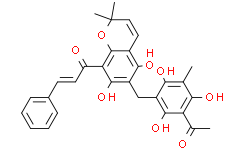
Chemical Properties
References
|
Return Policy
If you are in any way unsatisfied with your purchase, you may return any item(s) within 365 days of its original purchase date.
Please provide your Order Number in the email. We strive to reply to all email inquiries within one business day.
Tel: +86-21-58447131
Fax: +86-21-61642470 Email: sales@dcchemicals.com order@dcchemicals.com Website: www.dcchemicals.com |