| Cas No.: | 155877-83-1 |
| Chemical Name: | Benzoic acid,4-(7,8,9,10-tetrahydro-5,7,7,10,10-pentamethyl-5H-benzo[e]naphtho[2,3-b][1,4]diazepin-13-yl)- |
| Synonyms: | Benzoic acid,4-(7,8,9,10-tetrahydro-5,7,7,10,10-pentamethyl-5H-benzo[e]naphtho[2,3-b][1,4]diazepin-13-yl)-;LE 135;4-(7,8,9,10-TETRAHYDRO-5,7,7,10,10-PENTAMETHYL-5H-BENZO[E]NAPHTHO[2,3-B][1,4]DIAZEPIN-13-YL)BENZOIC ACID;4-(5,7,7,10,10-pentamethyl-8,9-dihydronaphtho[2,3-b][1,4]benzodiazepin-13-yl)benzoic acid;HMS3268H07;4-(7,8,9,10-Tetrahydro-5,7,7,10,10-pentamethyl-5H-benzo[e]naphtho[2,3-b][1,4]diazepin-13-yl)-benzoic acid;LE-135;4-(5,7,7,10,10-Pentamethyl-7,8,9,10-tetrahydro-5H-benzo[e]naphtho[2,3-b][1,4]diazepin-13-yl)benzoic acid;4-(7,8,9,10-Tetrahydro-5,7,7,10,10-pentamethyl-(5H)-benzo[e]naphtho[2.3-b][1.4]diazepine-13-yl)benzoicacid;Benzoic acid, 4-(7,8,9,10-tetrahydro-5,7,7,10,10-pentaMethyl-5H-benzo[e]naphtho[2,3-b][1,4]diazepin-13-yl)- |
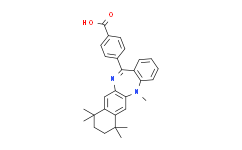
| SMILES: | CN1C2C=CC=CC=2C(C2C=CC(C(O)=O)=CC=2)=NC2C=C3C(=CC1=2)C(C)(C)CCC3(C)C |
| Formula: | C29H30N2O2 |
| M.Wt: | 438.560707569122 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | LE135 is a potent RAR antagonist that binds selectively to RARα (Ki of 1.4 μM) and RARβ (Ki of 220 nM), and has a higher affinity to RARβ. LE135 is highly selective over RARγ, RXRα, RXRβ and RXRγ. LE135 is also a potent TRPV1 and TRPA1 receptors activator with EC50s of 2.5 μM and 20 μM, respectively[1][2]. |
| Target: | RARβ:220 nM (Ki) RARα:1400 nM (Ki) TRPV1:2.5 μM (EC50) TRPA1:20 μM (EC50) |
| In Vivo: | LE135 provokes nociceptive responses and elicited thermal hyperalgesia mainly through TRPV1 channels, but required both TRPA1 and TRPV1 channels for producing mechanical allodynia. Intraplantar injection of LE135 (30 nmol/10 μL) induces mechanical hypersensitivity in wild-type and Trpa1−/− mice[2]. |
| In Vitro: | LE135 inhibits Am80-induced differentiation of human promyelocytic leukemia cells HL-60 with an IC50 of 150 nM[1]. LE135 inhibits retinoic acid (RA)-induced transcriptional activation of RARβ, but not RARα, RARγ or retinoid X receptor α (RXRα), on a variety of RA response elements. LE135 strongly represses 12-O-tetradecanoylphorbol-13-acetate-induced AP-1 activity in the presence of RARβ and RXRα[3]. |
| References: | [1]. H Umemiya, et al. Regulation of retinoidal actions by diazepinylbenzoic acids. Retinoid synergists which activate the RXR-RAR heterodimers. J Med Chem. 1997 Dec 19;40(26):4222-34. [2]. Shijin Yin, et al. LE135, a retinoid acid receptor antagonist, produces pain through direct activation of TRP channels. Br J Pharmacol. 2014 Mar;171(6):1510-20. [3]. Y Li, et al. Identification of a novel class of retinoic acid receptor beta-selective retinoid antagonists and their inhibitory effects on AP-1 activity and retinoic acid-induced apoptosis in human breast cancer cells. J Biol Chem. 1999 May 28;274(22):15360-6. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.