| Cas No.: | 480-10-4 |
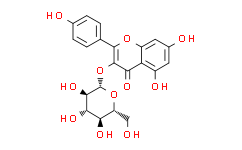
| Chemical Name: | Astragalin |
| Synonyms: | Astragalin;Kaempferol 3-O-beta-D-glucopyranoside;5,7-Dihydroxy-2-(4-hydroxyphenyl)-3-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one;4H-1-Benzopyran-4-one,3-(b-D-glucopyranosyloxy)-5,7-dihydroxy-2-(4-hydroxyphenyl)-;ASTRAGALIN(KAEMPFEROL-3-O-GLUCOSIDE)(AS) PrintBack;ASTRAGALIN(KAEMPFEROL-3-O-GLUCOSIDE)(P);Astragaline;Kaempferol 3-glucoside;KAEMPFEROL-3-GLUCOSIDE;Kaempferol-3-O-glucoside;STRAGALIN(KAEMPFEROL-3-O-GLUCOSIDE)(AS) PrintBack;3-Glucosylkaempferol;Astragaloside;Kaempferol 3-β-D-glucopyranoside;3,4′,5,7-Tetrahydroxyflavone 3-glucoside;3-(β-D-Glucopyranosyloxy)-5,7-dihydroxy-2-(4-methoxyphenyl)-4H-1-benzopyran-4-one;Kaempferol 3-O-glucoside;asragalin;Kaempferol-3-beta-monoglucoside;Kaempferol-3-D-glucoside;Kaempferol-3-beta-glucopyranoside;3,4',5,7-Tetrahydroxyflavone-3-glucoside;Kaempferol 3-O-beta-D-glucoside;APM8UQ3Z9O;4H-1-Benzopyran-4-one, 3-(beta-D-glucopyranosyloxy)-5,7-dihydroxy-2-(4-hydroxyphenyl)-;JPUKWEQWGBDDQB-QSOFNF |
| SMILES: | O1[C@]([H])([C@@]([H])([C@]([H])([C@@]([H])([C@@]1([H])C([H])([H])O[H])O[H])O[H])O[H])OC1C(C2=C(C([H])=C(C([H])=C2OC=1C1C([H])=C([H])C(=C([H])C=1[H])O[H])O[H])O[H])=O |
| Formula: | C21H20O11 |
| M.Wt: | 448.3769 |
| Purity: | >98% |
| Sotrage: | 4°C for 1 year, -20°C for more than 2 years |
| Description: | Astragalin (kaempferol-3-O-glucoside) is a flavonoid with anti-inflammatory activity and newly found in persimmon leaves and green tea seeds.IC50 value:Target: in vitro: Astragalin nontoxic at ≤ 20 μM suppressed cellular induction of Toll-like receptor 4 (TLR4) and ROS production enhanced by LPS. Both LPS and H2O2 induced epithelial eotaxin-1 expression, which was blocked by astragalin. LPS activated and induced PLCγ1, PKCβ2, and NADPH oxidase subunits of p22phox and p47phox in epithelial cells and such activation and induction were demoted by astragalin or TLR4 inhibition antagonizing eotaxin-1 induction. H2O2-upregulated phosphorylation of JNK and p38 MAPK was dampened by adding astragalin to epithelial cells, while this compound enhanced epithelial activation of Akt and ERK. H2O2 and LPS promoted epithelial apoptosis concomitant with nuclear condensation or caspase-3 activation, which was blunted by astragalin [1]. astragalin suppressed the expression of tumor necrosis factor α, interleukin 6, and nitric oxide in a dose-dependent manner in mMECs [2]. astragalin attenuated the infiltration of inflammatory cells, the activity of myeloperoxidase (MPO) and the expression of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β) in a dose-dependent manner. Additionally, Western blotting results showed that astragalin efficiently blunt decreased nuclear factor-kappaB (NF-κB) activation by inhibiting the degradation and phosphorylation of IκBα and the nuclear translocation of p65 [3]. Astragalin significantly reduced LPS-induced expression of iNOS, COX-2 and cytokines/chemokines, and production of NO in J774A.1 mouse macrophages. Astragalin inhibited LPSinduced activation of NF-κB as indicated by inhibition of degradation of IκBα, nuclear translocation of NF-κB, and NF-κB dependent gene reporter assay [4].in vivo: Mice were injected intraperitoneally (i.p.) with lipopolysaccharide (LPS) (dose range: 5-40 mg/kg). pretreatment with astragalin can improve survival during lethal endotoxemia and attenuate inflammatory responses in a murine model of lipopolysaccharide-induced acute lung injury [4]. |
| References: | [1]. Cho IH, et al. Astragalin inhibits airway eotaxin-1 induction and epithelial apoptosis through modulating oxidative stress-responsive MAPK signaling. BMC Pulm Med. 2014 Jul 29;14:122. [2]. Li F, et al. Inhibitory effects of astragalin on lipopolysaccharide-induced inflammatory response in mouse mammary epithelial cells. J Surg Res. 2014 Dec;192(2):573-81. [3]. Li F, et al. Astragalin suppresses inflammatory responses via down-regulation of NF-κB signaling pathway in lipopolysaccharide-induced mastitis in a murine model. Int Immunopharmacol. 2013 Oct;17(2):478-82. [4]. Kim MS, et al. Inhibitory effect of astragalin on expression of lipopolysaccharide-induced inflammatory mediators through NF-κB in macrophages. Arch Pharm Res. 2011 Dec;34(12):2101-7. [5]. Soromou LW, et al. Astragalin attenuates lipopolysaccharide-induced inflammatory responses by down-regulating NF-κB signaling pathway. Biochem Biophys Res Commun. 2012 Mar 9;419(2):256-61. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.