| Cas No.: | 129-46-4 |
| Chemical Name: | Suramin sodium salt |
| Synonyms: | Suramin sodium;8,8'-Carbonylbisimino-3,1-phenylenecarbonylimino-(4-methyl-3,1-phenylene)carbonyliminobis-1,3,5-naphthalenetrisulfonic acid hexasodium;Suramin hexasodium salt;Suramin (Sodium Salt);SURAMIN HEXASODIUM SALT(RG);BAY-205;hexasodium,8-[[4-methyl-3-[[3-[[3-[[2-methyl-5-[(4,6,8-trisulfonatonaphthalen-1-yl)carbamoyl]phenyl]carbamoyl]phenyl]carbamoylamino]benzoyl]amino]benzoyl]amino]naphthalene-1,3,5-trisulfonate;NF-060;Suramin sodium salt;Suramin. Hexasodium Salt;Suramine sodium salt;8,8'-[CARBONYLBIS[IMINO-3,1-PHENYLENECARBONYLIMINO(4-METHYL-3,1-PHENYLENE)CARBONYLIMINO]]BIS-1,3,5-NAPHTHALENETRISULFONIC ACID HEXASODIUM SALT;309f;nf060;F-309;bay205;naganin;moranyl;SURAMIN;Naganol;naganine;bayer205 |
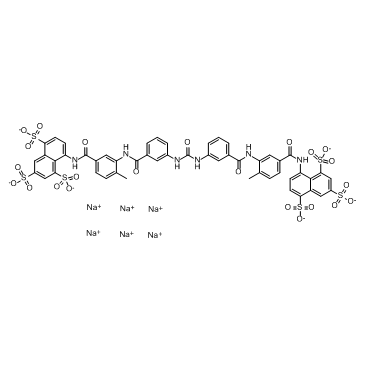
| SMILES: | O=C(NC1=CC(C(NC2=CC(C(NC3=CC=C(S(=O)([O-])=O)C4=CC(S(=O)([O-])=O)=CC(S(=O)([O-])=O)=C34)=O)=CC=C2C)=O)=CC=C1)NC5=CC(C(NC6=CC(C(NC7=CC=C(S(=O)([O-])=O)C8=CC(S(=O)([O-])=O)=CC(S(=O)([O-])=O)=C78)=O)=CC=C6C)=O)=CC=C5.[Na+].[Na+].[Na+].[Na+].[Na+].[Na+] |
| Formula: | C51H34N6Na6O23S6 |
| M.Wt: | 1429.17 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks4°C in DMSO,6 months-80°C in DMSO |
| Description: | Suramin sodium salt (BAY-205, NF-060) is an antitrypansomal drug that also possesses antitumor activity; inhibits CRL (Cullin-RING E3 ubiquitin ligases) activity by disrupting its ability to recruit Cdc34; induces accumulation of CRL substrates; also inhi |
| Target: | IC50: 5 μM (DNA topoisomerase II) |
| In Vivo: | Treatment with suramin shows lower values for pulmonary artery pressure, right ventricular hypertrophy, and distal vessel muscularization on day 21 compared to control rats. Suramin treatment suppresses PA-SMC proliferation and attenuates both the inflammatory response and the deposition of collagen. |
| In Vitro: | Suramin inhibits cell proliferation and DNA synthesis in cultured HeLa cells. The replication of SV40 DNA is completely abolished by 40 μM suramin. DNA polymerase α is sensitive to lower concentrations of suramin (IC50=8 µM) than is DNA polymerase δ (IC50=36 µM), whereas DNA polymerase β is relatively insensitive to the drug (IC50 of 90 µM)[1]. Suramin is a potent inhibitor of DNA strand exchange and ATPase activities of bacterial RecA proteins. Suramin inhibits RecA-catalysed proteolytic cleavage of the LexA repressor. The mechanism underlying such inhibitory actions of suramin involves its ability to disassemble RecA–single-stranded DNA filaments[2]. Suramin is a potent inhibitor of the nuclear enzyme DNA topoisomerase II. Suramin inhibits purified yeast topoisomerase II with an IC50 of about 5 μM. |
| Kinase Assay: | The ATPase assay is performed in a 10 μL reaction mixture containing 20 mM Tris-HCl (pH 7.5), 1 mM DTT, 8 mM MgCl2, 5 μM M13 circular ssDNA, 2.5 μM RecA from the specified bacterial species and increasing concentrations of suramin. The reaction is initiated by the addition of 2 mM [α-32P]ATP, incubated for 30 min at 37°C and stopped by the addition of 25 mM EDTA. |
| Animal Administration: | Rats: To assess the potential preventive and curative effects of suramin, rats are randomly divided into four groups after MCT injection. In the preventive strategy, the treatment is started on the first day, and one group receives 10 mg/kg suramin intravenously twice weekly for 3 weeks, while a second group receives only the vehicle at the same time points. To assess the potential curative effects of suramin, rats are given MCT and are left untreated for 21 days before being randomly divided into two groups that are subsequently treated with either suramin or vehicle from day 21 to day 42 inclusive. The effect of suramin on survival is evaluated from the day 21 of MCT injection to day 42 corresponding to the treatment period. |
| References: | [1]. Jindal HK, et al. Suramin affects DNA synthesis in HeLa cells by inhibition of DNA polymerases. Cancer Res. 1990 Dec 15;50(24):7754-7. [2]. Nautiyal A, et al. Suramin is a potent and selective inhibitor of Mycobacterium tuberculosis RecA protein and the SOS response: RecA as a potential target for antibacterial drug discovery. J Antimicrob Chemother. 2014 Jul;69(7):1834-43. [3]. Bojanowski K, et al. Suramin is an inhibitor of DNA topoisomerase II in vitro and in Chinese hamster fibrosarcomacells. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3025-9. [4]. Izikki M, et al. The beneficial effect of suramin on monocrotaline-induced pulmonary hypertension in rats. PLoS One. 2013 Oct 15;8(10):e77073. |