 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
| Cat. No. | Product Name | Field of Application | Chemical Structure |
|---|---|---|---|
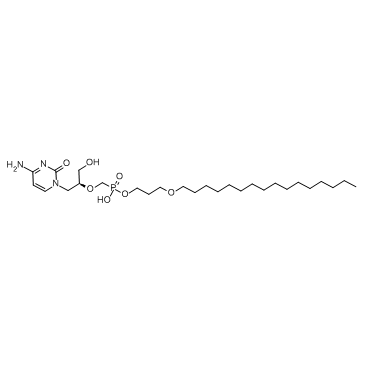
| DC7761 | Brincidofovir (CMX-001) Featured |
CMX001 (Brincidofovir; HDP-CDV) was developed as an orally active, lipophilic form of cidofovir (CDV); has enhanced activity in vitro and in vivo compared to CDV against certain herpesviruses, adenoviruses and orthopoxviruses.IC50 Value: 5.5 nM (EC50, in PDA at 7 dpi) [3]Target: anti-CMVCMX001 is currently in Phase II clinical studies for development as a therapeutic agent for human CMV, adenovirus and BK virus infections, as well as, for adverse events following smallpox vaccinations.in vitro: In PDA at 7 dpi, the CMX001 50% effective concentration (EC50) was 5.55 nM, the 50% cytotoxic concentration (CC50) was 184.6 nM, and the 50% selectivity index (SI50) was 33.3. The EC90 was 19.7 nM, the CC90 was 5,054 nM, and the SI90 was 256.1. In COS-7 cells, JCV replication was faster and the EC50 and EC90 were 18- and 37-fold higher than those in PDA, i.e., 0.1 μM and 0.74 μM (CC50, 0.67 μM; SI50, 6.7; CC90, 12.2 μM; SI90, 16.5) at 5 dpi [3].in vivo: CMX001 and CDV are equally efficacious at protecting mice from mortality following high ectromelia virus doses (10,000 x LD(50)) introduced by the intra-nasal route or small particle aerosol. Using CMX001 at a 10mg/kg dose followed by 2.5mg/kg doses every other-day for 14 days provided solid protection against mortality and weight loss following an intra-nasal challenge of (100-200) x LD(50) of ectromelia virus [1]. When CMX001 was administered orally to mice infected with HSV-1, mortality was reduced significantly (p≤0.001) with all three dose levels when treatments were initiated 24 h post viral inoculation. When treatments were started 48 h post viral inoculation, 5 and 2.5 mg/kg significantly reduced mortality (p≤ 0.001). If treatments were delayed until 72 h post viral inoculation, CMX001 did not reduce mortality or increase the mean day to death. When mice were infected intranasally with HSV-1 and treatments initiated 24 h post viral inoculation using CMX001 at 5 mg/kg or ACV at 100 mg/kg, virus replication in target organs was reduced by both CMX001 and ACV when compared to vehicle treated mice [2]. Toxicity: Diarrhea was the most common adverse event in patients receiving CMX001 at doses of 200 mg weekly or higher and was dose-limiting at 200 mg twice weekly. Myelosuppression and nephrotoxicity were not observed [4].
More description
|
|
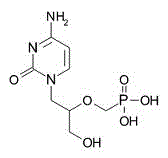
| DCAPI1021 | Cidofovir(Vistide) Featured |
Cidofovir(Vistide)
More description
|
|