| Cas No.: | 328543-09-5 |
| Chemical Name: | Imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one, 2-[4-[(dimethylamino)methyl]phenyl]-5,6-dihydro- |
| Synonyms: | AG14361;Imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one, 2-[4-[(dimethylamino)methyl]phenyl]-5,6-dihydro-;AG-14361;2-[4-[(Dimethylamino)methyl]phenyl]-5,6-dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one |
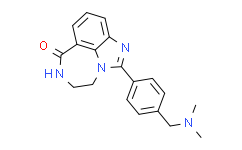
| SMILES: | O=C1NCCN2C3=C1C=CC=C3N=C2C4=CC=C(C=C4)CN(C)C |
| Formula: | C19H20N4O |
| M.Wt: | 320.3883 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | AG14361 is a potent PARP-1 inhibitor, with a Ki of < 5 nM, and in permeabilized SW620 and intact SW620 cells, the IC50s are 29 nM and 14 nM, respectively. |
| In Vivo: | AG14361 (5 and 15 mg/kg, i.p.) has no toxicity and does not inhibit the growth of tumor. However, AG14361 markedly enhances temozolomide activity against LoVo xenografts and delays tumor growth when combined with temozolomide. AG14361 (15 mg/kg, i.p.) treatment before irradiation dramaticly increases the sensitivity to radiation therapy of mice bearing LoVo xenografts[1]. AG14361 (30 mg/kg) synergizes lestaurtinib activity on inhibiting breast cancer tumors in allografts[2]. |
| In Vitro: | AG14361 is a potent PARP-1 inhibitor, with a Ki of < 5 nM, and in permeabilized SW620 and intact SW620 cells, the IC50s are 29 nM and 14 nM, respectively. AG14361 inhibits the proliferation of human cancer cells, such as A549, LoVo, and SW620 cells, with GI50s of 14 μM, 11.2 μM and 20 μM, respectively. Furthermore, AG14361 in combination with temozolomide markedly reduces the GI50 value of temozolomide in LoVo and A549 cells, but does not exert such an effect in SW620 cells[1]. AG14361 suppresses breast cancer cells with IC50s of 17 μM and 25 μM for 92 J-wt-BRCA1 and 92 J-sh-BRCA1 cells, respectively. AG14361 induces caspase 3/7 activation and cell cycle abnormalities, and also inhibits NF-κB signaling[2]. AG14361 (0.4 μM) enhances the growth-inhibitory and cytotoxic effects of topoisomerase I poisons, with no obvious effect on the formation and reversal of cleavable complexes, and increases the persistence of camptothecin-induced DNA single-strand breaks[3]. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.