| Cas No.: | 73069-14-4 |
| Chemical Name: | Atractylenolide II |
| Synonyms: | 2-Atractylenolide;Asterolide;atractylenolide II;AtractylenolideⅡ;(4aS,8aR,9aS)-3,8a-dimethyl-5-methylidene-4a,5,6,7,8,8a,9,9a-octahydronaphtho[2,3-b]furan-2(4H)-one;(4aS,8aR,9aS)-4a,5,6,7,8,8a,9,9a-Octahydro-3,8a-dimethyl-5-methylenenaphtho[2,3-b]furan-2(4H)-one;Atractylenolide-II;Atractylon-Autoxidationsprodukt A;Eudesmanolide;2,4,4a,5,6,7,8,8a,9,9a-Decahydro-3,8a-dimethyl-5-methylenenaphtho[2,3-b]furan-2-one;(4aS,8aR,9aS)-3,8a-dimethyl-5-methylidene-4a,6,7,8,9,9a-hexahydro-4H-benzo[f][1]benzofuran-2-one;3,8a-Dimethyl-5-methylidene-4a,6,7,8,9,9a-hexahydro-4H-benzo[f][1]benzofuran-2-one;Q63398440;HMS3886J22;BDBM50241946;N2205;S9314;X1091;C17886;069A144;(4aS,8aR,9aS)-3,8a-dimethyl-5-methylene-4a,5,6,7,8,8a,9,9a-octahydronaphtho[2,3-b]furan-2(4H)-one |
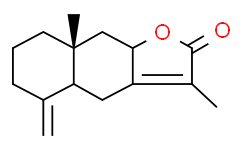
| SMILES: | O1C(C(C([H])([H])[H])=C2[C@]1([H])C([H])([H])[C@@]1(C([H])([H])[H])C([H])([H])C([H])([H])C([H])([H])C(=C([H])[H])[C@]1([H])C2([H])[H])=O |
| Formula: | C15H20O2 |
| M.Wt: | 232.3181 |
| Purity: | >98%, Standard References Grade |
| Sotrage: | 4°C for 1 year, -20°C for more than 2 years |
| Description: | Atractylenolide II is a sesquiterpene compound isolated from the dried rhizome of Atractylodes macrocephala (Baizhu in Chinese); anti-proliferative activity. |
| In Vivo: | Daily administration of AT-II (12.5, 25 mg/kg, i.g.) for 14 days significantly inhibited tumor growth in a B16 xenograft mouse model and inhibited the activation/phosphorylation of STAT3 and Src in the xenografts [2]. |
| In Vitro: | AT-II treatment for 48 h dose-dependently inhibited cell proliferation with an IC(50) of 82.3 μM, and induced G1 phase cell cycle arrest. Moreover, treatment with 75 μM AT-II induced apoptosis. These observations were associated with the decrease of the expression of Cdk2, phosphorylated-Akt, phosphorylated-ERK and Bcl-2, the increase of the expression of phosphorylated-p38, phosphorylated-p53, p21, p27, and activation of caspases-8, -9 and -3. In addition, a chemical inhibitor of p53, PFTα, significantly decreased AT-II-mediated growth inhibition and apoptosis [1]. In B16 and A375 cells, AT-II (20, 40 μm) treatment for 48 h dose-dependently reduced protein expression levels of phospho-STAT3, phospho-Src, as well as STAT3-regulated Mcl-1 and Bcl-xL. Overexpression of a constitutively active variant of STAT3, STAT3C in A375 cells diminished the antiproliferative and apoptotic effects of AT-II [2]. |
| References: | [1]. Ye Y, et al. Atractylenolide II induces G1 cell-cycle arrest and apoptosis in B16 melanoma cells. J Ethnopharmacol. 2011 Jun 14;136(1):279-82. [2]. Fu XQ, et al. Inhibition of STAT3 signalling contributes to the antimelanoma action of atractylenolide II. Exp Dermatol. 2014 Nov;23(11):855-7. |