| Cas No.: | 14513-15-6 |
| Chemical Name: | Cambinol |
| Synonyms: | 4(1H)-Pyrimidinone,2,3-dihydro-5-[(2-hydroxy-1-naphthalenyl)methyl]-6-phenyl-2-thioxo-;Cambinol;5-[(2-hydroxynaphthalen-1-yl)methyl]-6-phenyl-2-sulfanylidene-1H-pyrimidin-4-one;2 Inhibitor IV;2 Inhibitor IV, Cambinol;2-Mercapto-4-phenyl-5-(2-hydroxy-naphthyl-1-methyl)-6-hydroxy-pyrimidin;5-(2-hydroxy-naphthalen-1-ylmethyl)-6-phenyl-2-thioxo-2,3-dihydro-1H-pyrimidin-4-one;AC1MMYEF;NCIStruc1_001428;NCIStruc2_001159;NSC112546;SIRT1;SureCN3332758;NSC-1125476;5-(2-Hydroxynaphthalen-1-ylmethyl)-6-phenyl-2-thioxo-2,3-dihydro-1H-pyrimidin-4-one;5-[(2-hydroxy-1-naphthyl)methyl]-2-mercapto-6-phenyl-4(3H)-Pyrimidinone;Tetrahydro-5-[(2-hydroxy-1-naphthalenyl)methyl]-6-phenyl-2-thioxo-4(1H)-Pyrimidinone;5-[(2-Hydroxynaphthalen-1-yl)methyl]-6-phenyl-2-thioxo-2,3-dihydropyrimidin-4(1H)-one;5-[(2-Hydroxy-1-naphthyl)methyl]-6-phenyl-2-thioxo-2,3-dihydro-4(1H)-pyrimidinone;NSC-1125476, Tetrahydro-5-[(2-hydroxy-1-naphthalenyl)methyl]-6-phenyl-2-thioxo-4(1H)-Pyrimidinone, 5-(2-Hydroxynaphthalen-1-ylmethyl)-6-phenyl-2-thioxo-2,3-dihydro-1H-pyrimidin-4-one, 5-[(2-hydroxy-1-naphthyl)methyl]-2-mercapto-6-phenyl-4(3H)-Pyrimidinone;SIRT1/2 Inhibitor IV;SIRT1/2 Inhibitor IV, Cambinol;NSC-1125476, Tetrahydro-5-[(2-hydroxy-1-naphthalenyl)methyl]-6-phenyl-2-thioxo-4(1H)-Pyrimidinone, 5-(2-Hydroxynaphthalen-1-ylmethyl)-6-phenyl-2-thioxo-2,3-dihydro-1H-pyrimidin-4-one, 5-[(2-hydroxy-1- |
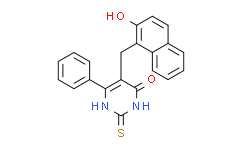
| SMILES: | S=C1N([H])C(C(=C(C2C([H])=C([H])C([H])=C([H])C=2[H])N1[H])C([H])([H])C1=C(C([H])=C([H])C2=C([H])C([H])=C([H])C([H])=C12)O[H])=O |
| Formula: | C21H16N2O2S |
| M.Wt: | 360.4289 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Cambinol is a SIRT1 and SIRT2 inhibitor with IC50 values of 56 and 59 μM, respectively. |
| In Vivo: | Cambinol is well tolerated in mice (100 mg/kg) and inhibits growth of Burkitt lymphoma xenografts. No significant weight loss occurs in cambinol-treated animals relative to controls. Inhibitors of NAD-dependent deacetylases may constitute novel anticancer agents[1]. |
| In Vitro: | Cambinol inhibits NAD-dependent deacetylase activity of human SIRT1 and SIRT2. Inhibition of SIRT1 activity with cambinol during genotoxic stress leads to hyperacetylation of key stress response proteins and promotes cell cycle arrest. Treatment of BCL6-expressing Burkitt lymphoma cells with cambinol as a single agent induces apoptosis, which is accompanied by hyperacetylation of BCL6 and p53. Cambinol has only weak inhibitory activity against SIRT5 (42% inhibition at 300 μM) and no activity against SIRT3[1]. |
| Cell Assay: | .The reporter construct with or without varying amounts of GAL4-BCL6 expression plasmid are introduced into NCI-H460 cells using calcium phosphate method. A plasmid containing cytomegalovirus (CMV)-driven β-galactosidase reporter (50 ng) is cotransfected to control for transfection efficiency. Sixteen hours after transfection, cells are treated with 100 μM cambinol of DMSO (control) for 24 hours and the luciferase and β-galactosidase activity is measured[1]. |
| Animal Administration: | Mice: Cambinolat the dose of 100 mg/kg, or vehicle are administered i.v. through tail vein injection or i.p. daily from day 5 to 19 (five injections per week). The dose of 100 mg/kg cambinol is the highest dose that could be administered as a single i.v. injection due to limited solubility of the drug. Tumor size is measured thrice a week using caliper and the tumor volumes are calculated[1]. |
| References: | [1]. Heltweg B, et al. Antitumor activity of a small-molecule inhibitor of human silent information regulator 2 enzymes. Cancer Res. 2006 Apr 15;66(8):4368-77. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.