| Cas No.: | 76081-98-6 |
| Chemical Name: | Imidazo[1,2-a]pyridine-3-acetonitrile,2-methyl-8-(phenylmethoxy)- |
| Synonyms: | Imidazo[1,2-a]pyridine-3-acetonitrile,2-methyl-8-(phenylmethoxy)-;2-(2-methyl-8-phenylmethoxyimidazo[1,2-a]pyridin-3-yl)acetonitrile;2-METHYL-8-(PHENYLMETHOXY)IMIDAZO[1,2-A]PYRIDINE-3-ACETONITRILE;SCH 28080;SCH 28080 NEW;[8-(benzyloxy)-2-methylimidazo[1,2-a]pyridin-3-yl]acetonitrile;2-Methyl-8-(phenylmethoxy)imidazo;2-methyl-8-(phenylmethoxy)-imidazo(1,2-A)-pyridine-3-acetonitrile;3-(cyanomethyl)-2-methyl-8-(phenylmethoxy)imidazopyridine;8-phenylmethoxy-2-methylimidazo[1,2-a]pyridine-3-acetonitrile;Imidazo(1,2-a)pyridine-3-acetonitrile, 2-methyl-8-(phenylmethoxy)-;Sch-28080 |
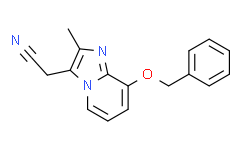
| SMILES: | N#CCC1=C(C)N=C2C(=CC=CN12)OCC1C=CC=CC=1 |
| Formula: | C17H15N3O |
| M.Wt: | 277.320503473282 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | SCH28080 inhibits gastric H+/K+-ATPase by K+-competitive binding, with an IC50 value of 20 nM in rabbit microsomal membranes[1]. Antisecretory and cytoprotective activities[2]. |
| Target: | IC50: 20 nM (H+/K+-ATPase, rabbit microsomal membranes)[1] |
| References: | [1]. Scott CK, et al. Studies on the mechanism of action of the gastric microsomal (H+ + K+)-ATPase inhibitors SCH 32651 and SCH 28080. Biochem Pharmacol. 1987 Jan 1;36(1):97-104. [2]. Long JF, et al. Gastric antisecretory and cytoprotective activities of SCH 28080. J Pharmacol Exp Ther. 1983 Jul;226(1):114-20. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.