| Cas No.: | 51146-57-7 |
| Chemical Name: | (R)-(-)-Ibuprofen |
| Synonyms: | Benzeneacetic acid, a-methyl-4-(2-methylpropyl)-, (aR)-;(R)-(-)-Ibuprofen;(alphaR)-alpha-Methyl-4-(2-methylpropyl)benzeneacetic acid;L-Ibuprofen;R-(-)-p-Isobutylhydratropic acid;R(-)-ibuprofen;CHEBI:47835;alphaR-Sethyl-4-(2-methylpropyl)benzeneacetic acid;(2R)-2-(4-isobutylphenyl)-propanoic acid;(2R)-2-(4-isobutylphenyl)propanoic acid;(-)-Ibuprofen;R-Ibuprofen;(R)-Ibuprofen;(R)-Ibuprofen |
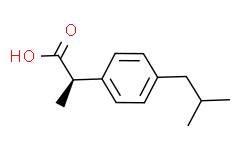
| SMILES: | C[C@@H](C(=O)O)C1C=CC(CC(C)C)=CC=1 |
| Formula: | C13H18O2 |
| M.Wt: | 206.280824184418 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | (R)-(-)-Ibuprofen is the R enantiomer of Ibuprofen, inactive on COX, inhibits NF-κB activation; (R)-(-)-Ibuprofen exhibits anti-inflammatory and antinociceptive effects. |
| Target: | NF-κB |
| In Vitro: | (R)-(-)-Ibuprofen is the R enantiomer of Ibuprofen, with no inhibitory effect on COX, but is involved in pathways of lipid metabolism and is incorporated into triglycerides along with endogenous fatty acids[1]. (R)-(-)-Ibuprofen (1 μM) significantly reduces NF-κB activation and completely prevents NF-κB induction at 10 μM. (R)-(-)-Ibuprofen inhibits NF-κB luciferase activity with an IC50 of 121.8 μM, weaker than that of S(+)-ibuprofen (IC50, 61.7 μM). Furthermore, (R)-(-)-Ibuprofen (10 mM) has no effect on HSF[2]. |
| References: | [1]. Evans AM, et al. Comparative pharmacology of S(+)-ibuprofen and (RS)-ibuprofen. Clin Rheumatol. 2001 Nov;20 Suppl 1:S9-14. [2]. Scheuren N, et al. Modulation of transcription factor NF-kappaB by enantiomers of the nonsteroidal drug ibuprofen. Br J Pharmacol. 1998 Feb;123(4):645-52. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.