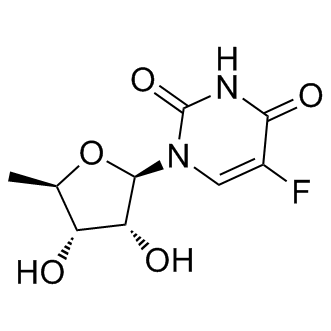
| Description: |
Doxifluridine(Ro 21-9738; 5'-DFUR) is a thymidine phosphorylase activator for PC9-DPE2 cells with IC50 of 0.62 μM.Doxifluridine is a fluoropyrimidine derivative and oral prodrug of the antineoplastic agent 5-fluorouracil (5-FU) with antitumor activity. Doxifluridine, designed to circumvent the rapid degradation of 5-FU by dihydropyrimidine dehydrogenase in the gut wall, is converted into 5-FU in the presence of pyrimidine nucleoside phosphorylase. 5-FU interferes with DNA synthesis and subsequent cell division by reducing normal thymidine production and interferes with RNA transcription by competing with uridine triphosphate for incorporation into the RNA strand.
in vitro: 5'-DFUR's metabolic product(N3-Me-5'-dFUR) was found to be non-toxic in all the cell growth experiments performed. The absence of cytotoxicity could be explained by the observation that the metabolite was not recognized as a substrate by thymidine phosphorilase, the enzyme responsible for 5-fluorouracil (5-FU) release from doxifluridine, as ascertained by high-performance liquid chromatography/ultraviolet (HPLC-UV) analysis of the incubation mixture.
in vivo: Administration of 200 mg of Furtulon to 23 beagle dogs, the plasma concentrations of doxifluridine, 5-FU, and 5-FUrd were measured simultaneously, using LC-MS/MS. The parent-metabolite compartment model with first-order absorption and Michaelis-Menten kinetics described the pharmacokinetics of doxifluridine, 5-FU, and 5-FUrd. Michaelis-Menten kinetics sufficiently explained the generation and elimination processes of 5-FU and 5-FUrd. |