| Cas No.: | 1346704-33-3 |
| Chemical Name: | N-[(6-Methyl-2-oxo-4-propyl-1H-pyridin-3-yl)methyl]-6-[2-(4-methylpiperazin-1-yl)pyridin-4-yl]-1-propan-2-ylindazole-4-carboxamide |
| Synonyms: | 1-(1-Methylethyl)-N-[(6-methyl-2-oxo-4-propyl-1,2-dihydro-3-pyridinyl)methyl]-6-[2-(4-methyl-1-piperazinyl)-4-pyridinyl]-1H-indazole-4-carboxamide;GSK343;1-(1-Methylethyl)-N-;1-(1-Methylethyl)-N-[(6-methyl-2-oxo-4-propyl-1,2-dihydro-3-pyridinyl)methyl]-6-[2-(4-methyl-1-piperazinyl)-4-pyridinyl...;1-(1-Methylethyl)-N-[(6-methyl-2-oxo-4-propyl-1,2-dihydro-3-pyridinyl)methyl]-6-[2-(4-methyl-1-piperazinyl)-4-pyridinyl]-1H-i;1-Isopropyl-N-((6-methyl-2-oxo-4-propyl-1,2-dihydropyridin-3-yl)methyl)-6-(2-(4-methylpiperazin-1-yl)pyridin-4-yl)-1H-indazole-4-carboxamide;1-Isopropyl-N-[(6-methyl-2-oxo-4-propyl-1,2-dihydro-3-pyridinyl)m ethyl]-6-[2-(4-methyl-1-piperazinyl)-4-pyridinyl]-1H-indazole-4-c arboxamide;GSK-343;N-[(1,2-Dihydro-6-methyl-2-oxo-4-propyl-3-pyridinyl)methyl]-1-(1-methylethyl)-6-[2-(4-methyl-1-piperazinyl)-4-pyridinyl]-1H-indazole-4-carboxamide;N-[(6-Methyl-2-oxo-4-propyl-1,2-dihydropyridin-3-yl)methyl]-6-[2-(4-methylpiperazin-1-yl)pyridin-4-yl]-1-(propan-2-yl)-1H-indazole-4-carboxamide;GSK 343;C31H39N7O2;compou;N-[(6-Methyl-2-oxo-4-propyl-1H-pyridin-3-yl)methyl]-6-[2-(4-methylpiperazin-1-yl)pyridin-4-yl]-1-pro |
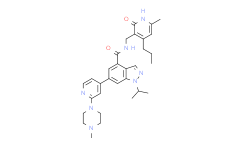
| SMILES: | O=C(C1C([H])=C(C([H])=C2C=1C([H])=NN2C([H])(C([H])([H])[H])C([H])([H])[H])C1C([H])=C([H])N=C(C=1[H])N1C([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])C1([H])[H])N([H])C([H])([H])C1C(N([H])C(C([H])([H])[H])=C([H])C=1C([H])([H])C([H])([H])C([H])([H])[H])= |
| Formula: | C31H39N7O2 |
| M.Wt: | 541.6871 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | GSK343 is a highly potent and selective EZH2 inhibitor with an IC50 of 4 nM. |
| In Vivo: | Compare with the controls, GSK343 (5 mg/kg)-treated mice exhibits significantly inhibited tumor growth. The average tumor volume and weight of the GSK343-treated cohort is remarkably reduced. As early as 20 days post-implantation, a significant reduction in tumor growth is observed in the GSK343-treated cohort relative to the control cohort; this difference persisted through the remainder of the study. In addition, compare with the control cohort, the GSK343-treated animals in the xenograft model show a remarkable increase in messenger RNA levels of E-cadherin but a significant decrease in vimentin messenger RNA levels[2]. |
| In Vitro: | GSK343, which contains an n-propyl group at the 4-position of the pyridone, has EZH2 Kiapp=1.2±0.2 nM. In this 6-day proliferation assay, among the cell lines evaluated in this study, the prostate cancer cell line LNCaP is the most sensitive to EZH2 inhibition, with growth IC50 value of 2.9 μM for GSK343[1]. GSK343 is found to have half maximal inhibitory concentration values of 13 μM in HeLa cells and 15 μM in SiHa cells[2]. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.