| Cas No.: | 1207456-01-6 |
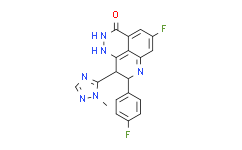
| Chemical Name: | Talazoparib |
| Synonyms: | BMN673;(8S,9R)-5-Fluoro-8-(4-fluorophenyl)-9-(1-methyl-1H-1,2,4-triazol-5-yl)-8,9-dihydro-2H-pyrido[4,3,2-de]phthalazin-3(7H)-one;(8S,9R)-BMN 673;(BMN 673);BMN-673;Talazoparib (BMN 673);BMN673,BMN-673;LT-673;Talazoparib;BMN 673;9QHX048FRV;(8S,9R)-5-Fluoro-8-(4-fluorophenyl)-2,7,8,9-tetrahydro-9-(1-methyl-1H-1,2,4-triazol-5-yl)-3H-pyrido[4,3,2-de]phthalazine-3-one;(8s,9r)-5-Fluoro-8-(4-Fluorophenyl)-9-(1-Methyl-1h-1,2,4-Triazol-5-Yl)-2,7,8,9-Tetrahydro-3h-Pyrido[4,3,2-De]phthalazin-3-One;Talazoparib [USAN:IN |
| SMILES: | FC1=C([H])C2C(N([H])N=C3C=2C(=C1[H])N([H])[C@]([H])(C1C([H])=C([H])C(=C([H])C=1[H])F)[C@@]3([H])C1=NC([H])=NN1C([H])([H])[H])=O |
| Formula: | C19H14F2N6O |
| M.Wt: | 380.35 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Talazoparib (BMN-673) is a highly potent PARP1/2 inhibitor with Kis of 1.2 nM and 0.87 nM, respectively. |
| In Vivo: | Talazoparib (BMN 673; 1 mg/kg, p.o.) is orally available, displaying favorable pharmacokinetic (PK) properties and remarkable antitumor efficacy in the BRCA1 mutant MX-1 breast cancer xenograft model following oral administration as a single-agent or in combination with chemotherapy agents such as temozolomide and cisplatin[1]. Talazoparib (BMN 673) is readily orally bioavailable, with more than 40% absolute oral bioavailability in rats when dosed in carboxylmethyl cellulose. Oral administration of Talazoparib elicits remarkable antitumor activity, xenografted tumors that carry defects in DNA repair due to BRCA mutations or PTEN deficiency are profoundly sensitive to oral Talazoparib treatment at well-tolerated doses in mice[2]. |
| In Vitro: | Talazoparib (BMN 673) demonstrates excellent potency, inhibiting PARP1 and PARP2 enzyme activity with Ki=1.2 and 0.87 nM, respectively[1]. Talazoparib (BMN 673) exhibits selective antitumor cytotoxicity and elicits DNA repair biomarkers at much lower concentrations than earlier generation PARP1/2 inhibitors (such as Olaparib, Rucaparib, and Veliparib)[2]. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.