| Cas No.: | 64907-22-8 |
| Chemical Name: | Cholest-5-ene-3,7,25-triol,(3b,7a)- |
| Synonyms: | Cholest-5-ene-3,7,25-triol,(3b,7a)-;3β,7α,25-Trihydroxycholest-5-ene;3β,7α,25-Trihydroxyc;7α,25-dihydroxy Cholesterol;25-DIHYDROXYCHOLESTEROL;3b,7a,25-Trihydroxycholest-5-ene;7a,25-Dihydroxycholesterol;5-Cholesten-3β,7α,25-triol;cholest-5-ene-3β,7α,25-triol |
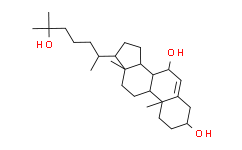
| SMILES: | OC1C=C2C(C)(CCC(O)C2)C2C1C1C(C)(CC2)C(C(C)CCCC(O)(C)C)CC1 |
| Formula: | C27H46O3 |
| M.Wt: | 418.652348995209 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | 7α, 25-dihydroxycholesterol (7α,25-OHC) is a potent and selective agonist and endogenous ligand of the orphan GPCR receptor EBI2 (GPR183). 7α, 25-dihydroxycholesterol is highly potent at activating EBI2 (EC50=140 pM; Kd=450 pM). 7α, 25-dihydroxycholesterol can serve as a chemokine directing migration of B cells, T cells and dendritic cells[1][2]. |
| Target: | Human Endogenous Metabolite |
| In Vivo: | EBI2-deficient B cells or normal B cells desensitized by 7α,25-Dihydroxycholesterol (1 μM; 1.5 hours) pre-treatment shows reduced homing to follicular areas of the spleen[1]. |
| In Vitro: | In vitro, 7α, 25-dihydroxycholesterol (7α,25-OHC) stimulates the migration of EBI2-expressing mouse B and T cells with half-maximum effective concentration values around 500 pM, but had no effect on EBI2-deficient cells[1]. |
| References: | [1]. Liu C, et al. Oxysterols direct B-cell migration through EBI2. Nature. 2011 Jul 27;475(7357):519-23. [2]. Hannedouche S, et al. Oxysterols direct immune cell migration via EBI2. Nature. 2011 Jul 27;475(7357):524-7. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.