| Cas No.: | 248281-84-7 |
| Chemical Name: | Laquinimod |
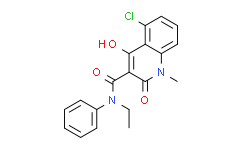
| Synonyms: | 5-Chloro-N-ethyl-4-hydroxy-1-methyl-2-oxo-N-phenyl-1,2-dihydroquinoline-3-carboxamide;Laquinimod;5-chloro-N-ethyl-4-hydroxy-1-methyl-2-oxo-N-phenylquinoline-3-carboxamide;CIVENTICHEM CV-4057;Lanquinimod;LAQUINIMOD, CIVENTICHEM CV-4057, 5-CHLORO-4-HYDROXY-1-METHYL-2-OXO-1,2-DIHYDROQUINOLINE-3-CARBOXYLIC ACID ETHYL PHENYLA...;5-chloro-1,2-dihydro-N-ethyl-4-hydroxy-1-methyl-2-oxo-N-phenyl-3-quinoline carboxamide;5-Chloro-4-hydro;5-Chloro-N-Et-4-hydroxy-1-methyl-2-oxo-N-Ph-1,2-dihydroquinoline-3-carboxamide;5-chloro-N-ethyl-1,2-dihydro-4-hydroxy-1-methyl-2-oxo-N-phenyl-3-quinolinecarboxamide;5-chloro-n-ethyl-2-hydroxy-1-methyl-4-oxo-n-phenyl-1,4-dihydroquinoline-3-carboxamide;ABR 215062;ABR-215062;5-Chloro-4-hydroxy-1-methyl-2-oxo-N-ethyl-N-phenyl-1,2-dihydroquinoline-3-carboxamide;5-Chloro-4-hydroxy-1-methyl-2-oxo-1,2-dihydro-quinoline-3-carboxylic acid ethyl-phenyl-amide;908SY76S4G;N-Ethyl-N-phenyl-1,2-dihydro-4-hydroxy-5-chloro-1-methyl-2-oxoquinoline-3-carboxamide;C19H17ClN2O3;5-chloro-N-ethyl-4-hydroxy- |
| SMILES: | ClC1=C([H])C([H])=C([H])C2=C1C(=C(C(N(C1C([H])=C([H])C([H])=C([H])C=1[H])C([H])([H])C([H])([H])[H])=O)C(N2C([H])([H])[H])=O)O[H] |
| Formula: | C19H17ClN2O3 |
| M.Wt: | 356.8029 |
| Purity: | 99% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Laquinimod is a potent immunomodulator which prevents neurodegeneration and inflammation in the central nervous system. |
| In Vivo: | Laquinimod treatment inhibits donor myelin-specific T cells from transferring EAE to naive recipient mice. In vivo laquinimod treatment alters subpopulations of myeloid antigen presenting cells (APC) that include a decrease in CD11c+CD11b+CD4+ dendritic cells (DC) and an elevation of CD11bhiGr1hi monocytes[1]. |
| In Vitro: | Laquinimod reverses EAE and inhibits pathogenic T cell immune responses. Laquinimod reverses RR-EAE and inhibits inflammatory T cell responses via a direct effect on myeloid APC. Laquinimod alters myeloid APC subsets and inhibits Th1 and Th17 polarization of myelin-specific T cells. Laquinimod-induced type II (M2) monocytes reverse established EAE[1]. Laquinimod modulates the phenotype of B cells of healthy donors. Laquinimod modulates expression of markers related to regulatory capacity in B cells of RRMS patients. Laquinimod reduces IFNγ cytokine expression in CD4+ T cells[2]. |
| Cell Assay: | Purified CD11b+ cells from laquinimod- or vehicle-treated mice are cultured with naive CD4+ cells isolated from laquinimod- or vehicle-treated 2D2 mice and antigen (MOG p35-55, 20 µg/mL). Cells are cultured in 96-well microtitre plates at a concentration of 0.25×106 cells/mL. Culture medium consisted of RPMI 1640 supplemented with L-glutamine (2 mM), sodium pyruvate (1 mM), penicillin (100 U/mL), streptomycin (0.1 mg/mL), 2-mercaptoethanol (5×10-5 M) and 10% (v/v) fetal bovine serum. Cells are incubated for 48 h and pulsed for 18 h with 1 µCi per well of [3H]-thymidine before harvesting. |
| Animal Administration: | Seven to 10-week-old female C57BL/6, DBA/1 or SJL/J mice are injected subcutaneously with 50 µg MOG p35-55, 50 µg rMOG or 100 µg PLP p139-151, respectively, in complete Freund's adjuvant. After immunization and 2 days later, mice receive 200 ng (C57BL/6) or 100 ng (SJL/J) pertussis toxin intraperitoneally (i.p.). For adoptive transfer, donor SJL/J mice are immunized as described above and treated daily with laquinimod or vehicle. 10 days later, cells from draining lymph nodes and spleen are isolated, re-stimulated for 48 h (20 µg/mL PLP p139-151), and injected i.p. into naive SJL/J recipients (107 cells per mouse). Animals are observed daily and clinical scores are assessed as follows: 0, no signs; 1, decreased tail tone; 2, mild monoparesis or paraparesis; 3, severe paraparesis; 4, paraplegia and/or quadraparesis; and 5, moribund or death. |
| References: | [1]. Schulze-Topphoff, Ulf., et al. Laquinimod, a quinoline-3-carboxamide, induces type II myeloid cells that modulate central nervous system autoimmunity. PLoS One (2012), 7(3), e33797. [2]. Toubi E, et al. Laquinimod modulates B cells and their regulatory effects on T cells in Multiple Sclerosis. J Neuroimmunol. 2012 Oct 15;251(1-2):45-54. [3]. Brück W, et al. Reduced astrocytic NF-κB activation by laquinimod protects from cuprizone-induced demyelination. Acta Neuropathol. 2012 Sep;124(3):411-24. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.