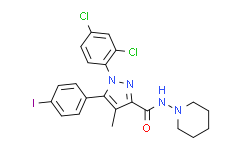
| Description: |
AM251 is a selective cannabinoid 1 (CB1) receptor antagonist with an IC50 of 8 nM, also acts as a potent GPR55 agonist with an EC50 of 39 nM. |
| Target: |
IC50: 8 nM (CB1 receptor)[1] |
| In Vivo: |
The CB1 antagonist AM251 (3 mg/kg, i.p.) decreases capsaicin-evoked nocifensive behavior (F1,18=28.45, p<0.0001). This suppressive effect is genotype dependent (F1,18=14.83, p<0.01), and the interaction between the effects of genotype and AM251 approached significance (F1,18=4.704, p=0.0587). Planned comparisons reveal that AM251 reduces nocifensive behaviors in fatty-acid amide hydrolase (FAAH) KO mice (p<0.01) but fails to alter nocifensive behavior in WT mice (p>0.2) relative to their respective vehicle controls. AM251 (3 mg/kg, i.p.) reduces the duration of heat hypersensitivity in FAAH KO (F1,9=21.43, p<0.01) but not WT mice (p>0.3). AM251 suppresses capsaicin-evoked heat hypersensitivity in a time-dependent manner in FAAH KO (F5,9=4.349, p<0.01) but not in WT mice (p>0.3). Post-hoc analysis reveals that FAAH KO mice receiving vehicle (i.p.) display heightened thermal hypersensitivity at 30 (p<0.05), 60 (p<0.05), and 90 (p<0.001) minutes post-capsaicin in comparison to FAAH KO animals receiving AM251)[4]. One-way ANOVA shows that AM251 (AM-251) injected into the rats significantly decreases both of the percentage of entries in the open arms and time spent in the open arms, compare to controls. The Tukey-Kramer test analysis reveals a significant reduction for the doses of 1 mg/kg (P<0.05) and 5 mg/kg (P<0.01) compare to control rats in the time spent in the open arms. Also, AM251 significantly decreases percentage of entries in the open arms for the doses of 1 and 5 mg/kg (P<0.05)[5]. |
| In Vitro: |
AM251 is a CB1 receptor antagonist/inverse agonist. AM251 produces an agonist response in HEK293 cells, similar to that found in the yeast expression system[2]. AM-251 reduces cholesteryl ester synthesis in unstimulated and acetylated LDL-stimulated Raw 264.7 macrophages, CB2+/+ and CB2-/- peritoneal macrophages[3]. |
| Kinase Assay: |
Macrophages are seeded (2×106/well) in 12-well culture plates. AM-251 or SR144528 are added from 4 mM stock solutions prepared in DMSO, 1h prior to the addition of 7-ketocholesterol (7KC) from a 2 mg/mL ethanol stock solution. Controls are adjusted to receive equivalent volumes of DMSO and ethanol. After 16 h, caspase-3 activity is determined. All treatments are done in triplicate and the data presented as the mean RFLU/mg protein±SD[3]. |
| Animal Administration: |
Mice[4] A total of 246 mice weighing 17-48 g are used in these experiments. Following determination of baseline responding, mice receive a single i.p injection (5 mL/kg) of AMG9810 (3 mg/kg, n=5 per group), AM251 (3 mg/kg, n=5 per group), or vehicle (n=6 per group). I.p. injections are performed 30 min prior to i.pl. capsaicin or vehicle administration. Paw withdrawal latencies are assessed before and 10, 30, 60, 90 and 120 min after intradermal injection of capsaicin or vehicle. Paw withdrawal latencies are measured in duplicate in each paw at each time point, and are reported as the mean of the two duplicate determinations from each animal, averaged across subjects. Rats[5] Male Wistar rats weighting 250-350 are used.The following agents are used: CB1 receptor agonist, Win-55212 (0.3, 1 and 5 mg/kg, i.p. ); CB1 receptor antagonist, AM251 (0.3, 1 and 5 mg/kg, i.p.); endocannabinoid breakdown inhibitor, URB-597 (0.03, 0.1 and 0.3 mg/kg, i.p.) in the study. Physiological saline (0.9% sodium chloride) is used as the vehicle. All drugs are prepared freshly and administered intraperitoneally (i.p.) in a volume of 0.1 mL per 10 g of body weight of the rats. All substances are dissolved in physiological saline and are administrated 30 min before elevated plus-maze test. |
| References: |
[1]. Bruno A, et al. Beyond radio-displacement techniques for identification of CB1 ligands: the first application of afluorescence-quenching assay. Sci Rep. 2014 Jan 20;4:3757.
[2]. Sharir H, et al. Pharmacological characterization of GPR55, a putative cannabinoid receptor. Pharmacol Ther. 2010 Jun;126(3):301-13.
[3]. Thewke D, et al. AM-251 and SR144528 are acyl CoA:cholesterol acyltransferase inhibitors. Biochem Biophys Res Commun. 2009 Apr 3;381(2):181-6.
[4]. Carey LM, et al. A pro-nociceptive phenotype unmasked in mice lacking fatty-acid amide hydrolase. Mol Pain. 2016 May 13;12. pii: 1744806916649192.
[5]. Komaki A, et al. Study the Effect of Endocannabinoid System on Rat Behavior in Elevated Plus-Maze. Basic Clin Neurosci. 2015 Jul;6(3):147-53.
[6]. Jiang X, et al. Role of cannabinoid receptor type 1 in tibial and pudendal neuromodulation of bladder overactivity in cats. Am J Physiol Renal Physiol. 2017 Mar 1;312(3):F482-F488.
[7]. Sun L, et al. Endocannabinoid activation of CB1 receptors contributes to long-lasting reversal of neuropathic pain by repetitive spinal cord stimulation. Eur J Pain. 2017 May;21(5):804-814. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.