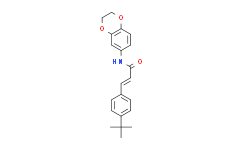
| Cas No.: | 545395-94-6 |
| Chemical Name: | (E)-3-(4-t-Butylphenyl)-N-(2,3-dihydrobenzo(b)(1,4)dioxin-6-yl)acrylamide |
| Synonyms: | 2-Propenamide,N-(2,3-dihydro-1,4-benzodioxin-6-yl)-3-[4-(1,1-dimethylethyl)phenyl]-, (2E)-;(2E)-N-(2,3-DIHYDRO-1,4-BENZODIOXIN-6-YL)-3-[4-(1,1-DIMETHYLETHYL)PHENYL]-2-PROPENAMIDE;AMG 9810;AMG 9810,(2E)-N-(2,3-Dihydro-1,4-benzodioxin-6-yl)-3-[4-(1,1-dimethylethyl)phenyl]-2-propenamide;AMG-9810;2E-N-(2,3-dihydro-1,4-benzodioxin-6-yl)-3-[4-(1,1-dimethylethyl)phenyl]-2-Propenamide;AMG9810;182HIJ2D7F;(2E)-3-(4-tert-butylphenyl)-N-(2,3-dihydro-1,4-benzodioxin-6-yl)prop-2-enamide;trans-cinnamide;(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo(b)(1,4)dioxin-6-yl)acrylamide;GTPL6347;BDBM20510 |
| SMILES: | O1C([H])([H])C([H])([H])OC2C([H])=C([H])C(=C([H])C1=2)N([H])C(/C(/[H])=C(\[H])/C1C([H])=C([H])C(=C([H])C=1[H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H])=O |
| Formula: | C21H23NO3 |
| M.Wt: | 337.4122 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | AMG9810 is a selective and competitive vanilloid receptor 1 (TRPV1) antagonist with IC50 values of 24.5 and 85.6 nM for human and rat TRPV1, repectively. |
| In Vivo: | AMG9810 is effective at preventing capsaicin-induced eye wiping in a dose-dependent manner, and it reverses thermal and mechanical hyperalgesia in a model of inflammatory pain induced by intraplantar injection of complete Freund's adjuvant. At effective doses, AMG9810 does not show any significant effects on motor function. AMG9810 is the first cinnamide TRPV1 antagonist reported to block capsaicin-induced eye wiping behavior and reverse hyperalgesia in an animal model of inflammatory pain[1]. AMG9810, promotes mouse skin tumor development. The topical application of AMG9810 results in a significant increase in the expression level of the epidermal growth factor receptor (EGFR) and its downstream Akt/mammalian target of rapamycin (mTOR)-signaling pathway[2]. |
| In Vitro: | AMG9810 is a competitive antagonist of capsaicin activation (IC50 value for human TRPV1, 24.5±15.7 nM; rat TRPV1, 85.6±39.4 nM) and blocks all known modes of TRPV1 activation, including protons (IC50 value for rat TRPV1, 294±192 nM; human TRPV1, 92.7±72.8 nM), heat (IC50 value for rat TRPV1, 21±17 nM; human TRPV1, 15.8±10.8 nM), and endogenous ligands, such as anandamide, N-arachidonyl dopamine, and oleoyldopamine. AMG9810 blocks capsaicin-evoked depolarization and calcitonin gene-related peptide release in cultures of rat dorsal root ganglion primary neurons. AMG9810 inhibits capsaicin-, proton-, heat-, and endogenous ligand-induced uptake of 45Ca2+ into TRPV1-expressing cells[1]. |