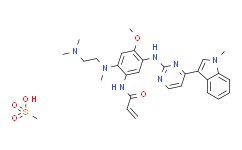
| Cas No.: | 1421373-66-1 |
| Chemical Name: | Osimertinib mesylate |
| Synonyms: | AZD-9291 (Mesylate);N-[2-[[2-(Dimethylamino)ethyl]methylamino]-4-methoxy-5-[[4-(1-methyl-1H-indol-3-yl)-2-pyrimidinyl]amino]phenyl]-2-propenamide methanesulfonate (1:1);AZD-9291 mesylate N-[2-[[2-(Dimethylamino)ethyl]methylamino]-4-methoxy-5-[[4-(1-methyl-1H-indol-3-yl)-2-pyrimidinyl]amino]phenyl]-2-propenamide methanesulfonate (1:1);Mereletinib mesylate;Osimertinib;N-[2-(2-dimethylaminoethylmethylamino)-4-methoxy-5-[[4-(1-methylindol-3-yl)pyrimidin-2-yl]amino]phenyl]prop-2-enamide mesylate salt;Osimertinib Mesylate(AZD9291);AZD9291 Mesylate;2-Propenamide, N-[2-[[2-(dimethylamino)ethyl]methylamino]-4-methoxy-5-[[4-(1-methyl-1H-indol-3-yl)-2-pyrimidinyl]amino]phenyl]-, methanesulfonate (1:1);AZD9291;AZD-9291;AZD-9291 mesylate;Osimertinib mesylate;(Mesylate);N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-((4-(1-methyl-1H-indol-3-yl)pyrimidin-2-yl)amino)phenyl)acrylamide methanesulfonate;N-[2-[[2-(Dimethylamino)ethyl]methylamino]-4-methoxy-5-[[4-(1-methyl-1H-indol-3-yl)-2-pyrimidinyl]amino]phenyl]-2-propenamide methanesulfonate;N-[2-[[2-(Dimethylamino)ethyl]methylamino]-4-methoxy-5-[[4-(1-methyl-1H-indol-3-yl)-2-pyrimidinyl]amino]phenyl]-2-propenamide methanesulfonate. |
| SMILES: | C=CC(NC1=CC(NC2=NC=CC(C3=CN(C)C4=C3C=CC=C4)=N2)=C(OC)C=C1N(CCN(C)C)C)=O.CS(=O)(O)=O |
| Formula: | C29H37N7O5S |
| M.Wt: | 595.72 |
| Purity: | >99% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Osimertinib mesylate (AZD-9291 mesylate) is an irreversible and mutant selective EGFR inhibitor with IC50s of 12 and 1 nM against EGFRL858R and EGFRL858R/T790M, respectively. |
| In Vitro: | Osimertinib (AZD-9291) shows similar potency to early generation tyrosine kinase inhibitor (TKIs) in inhibiting EGFR phosphorylation in EGFR cells harboring sensitising EGFR mutants including PC-9 (ex19del), H3255 (L858R) and H1650 (ex19del), with mean IC50 values ranging from 13 to 54 nM for Osimertinib. Osimertinib (AZD-9291) also potently inhibits phosphorylation of EGFR in T790M mutant cell lines (H1975 (L858R/T790M), PC-9VanR (ex19del/T790M), with mean IC50 potency less than 15 nM[1]. |
| Cell Assay: | PC-9 cells are seeded into T75 flasks (5×105 cells/flask) in RPMI growth media and incubated at 37°C, 5% CO2. The following day the media is replaced with media supplemented with a concentration of EGFR inhibitor equal to the EC50 concentration predetermined in PC-9 cells. Media changes are carried out every 2-3 days and resistant clones allowed to grow to 80% confluency prior to the cells being trypsinised and reseeded at the original seeding density in media containing twice the concentration of EGFR inhibitor. Dose escalations are continued until a final concentration of 1.5 μM Gefitinib, 1.5 μM Afatinib, 1.5 μM WZ4002 or 160 nM Osimertinib (AZD-9291) are achieved[1]. |
| Animal Administration: | Mice[1] The EGFRL858R and EGFRL858R+T790M mice (male and female) are used. Osimertinib (AZD-9291) is suspended in 1% Polysorbate 80 and administered via oral gavage once daily at the doses of 7.5 mg/kg and 5 mg/kg, respectively. Mice are imaged weekly at the Vanderbilt University Institute of Imaging Science. For immunoblot analysis, mice are treated for eight hours with drug as described before dissection and flash freezing of the lungs. Lungs are pulverized in liquid nitrogen before lysis. Rats[2] The male RccHan:WIST rats (10-week-old) are received a single oral dose of Osimertinib (200 mg/kg). Blood glucose levels are measured using an Accuchek Active meter. Serum insulin concentrations are determined using a commercial rat ELISA kit. |
| References: | [1]. Cross DA, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014 Sep;4(9):1046-61. [2]. Finlay MR, et al. Discovery of a potent and selective EGFR inhibitor (AZD9291) of both sensitizing and T790M resistancemutations that spares the wild type form of the receptor. J Med Chem. 2014 Oct 23;57(20):8249-67. [3]. Yasumuro O, et al. Changes in gefitinib, erlotinib, and osimertinib pharmacokinetics under various gastric pH levels following oral administration of omeprazole and vonoprazan in rats. Xenobiotica. 2017 Nov 17:1-7. [4]. Yoshimi Ohashi, et al. Targeting the Golgi apparatus to overcome acquired resistance of non-small cell lung cancer cells to EGFR tyrosine kinase inhibitors. Oncotarget. 2017 Dec 6;9(2):1641-1655. |