| Cas No.: | 934162-61-5 |
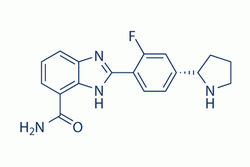
| Chemical Name: | 2-(2-fluoro-4-((S)-pyrrolidin-2-yl)phenyl)-3H-benzo[d]imidazole-4-carboxamide |
| Synonyms: | A966492,A 966492,A-966492 |
| SMILES: | FC1=CC([C@H]2NCCC2)=CC=C1C3=NC4=C(C(N)=O)C=CC=C4N3 |
| Formula: | C18H17FN4O |
| M.Wt: | 324.35 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | A-966492 is a novel and potent inhibitor of PARP1 and PARP2 with Ki of 1 nM and 1.5 nM, respectively. |
| Target: | PARP-1:1 nM (Ki) PARP-2:1.5 nM (Ki) |
| In Vivo: | A-966492 demonstrates good in vivo efficacy in a B16F10 subcutaneous murine melanoma model in combination with temozolomide and in an MX-1 breast cancer xenograft model both as a single agent and in combination with carboplatin. In addition, A-966492 has excellent pharmaceutical properties and has demonstrated in vivo efficacy in preclinical mouse tumor models in combination with TMZ and carboplatin, as well as single agent activity in a BRCA1-deficient MX-1 tumor model. A-966492 is further characterized in Sprague−Dawley rats, beagle dogs, and cynomolgus monkeys, with A-966492 demonstrating oral bioavailabilities of 34−72% and half-lives of 1.7−1.9 hours. In vivo, A-966492 demonstrates significant enhancement of the efficacy of TMZ in a murine B16F10 syngeneic melanoma model, with the A-966492 combination groups showing superior efficacy[1]. |
| In Vitro: | A-966492 is one of the most potent PARP inhibitors. A-966492 displays excellent potency against the PARP-1 enzyme with a Ki of 1 nM and an EC50 of 1 nM in a whole cell assay. A-966492 significantly enhances the efficacy of TMZ in a dose-dependent manner. In addition, A-966492 is orally bioavailable across multiple species, crosses the blood−brain barrier, and appears to distribute into tumor tissue. A-966492 represents a promising, structurally diverse benzimidazole analogue and is being further characterized preclinically[1]. |
| Kinase Assay: | The enzyme assay is conducted in buffer containing 50 mM Tris, pH 8.0, 1 mM dithiothreitol(DTT), and 4 mM MgCl2. PARP reactions contains 1.5 μM [3H]-NAD+ (1.6 μCi/mmol), 200 nM biotinylated histone H1, 200 nM slDNA, and 1 nM PARP-1 or 4 nM PARP-2 enzyme. Autoreactions utilizing SPA bead-based detection are carried out in 100 μL volumes in white 96-well plates. Reactions are initiated by adding 50 μL of 2X NAD+ substrate mixture to 50 μL of 2× enzyme mixture containing PARP and DNA. These reactions are terminated by the addition of 150 μL of 1.5 mM benzamide (appr 1×103-fold over its IC50). A 170 μL amount of the stopped reaction mixtures is transferred to streptavidin-coated Flash Plates, incubated for 1 hour, and counted using a TopCount microplate scintillation counter. Ki data are determined from inhibition curves at various substrate concentrations. |
| Cell Assay: | C41 cells are treated with A-966492 for 30 minutes in a 96-well plate. PARP are activated by damaging DNA with 1 mM H2O2 for 10 minutes. Cells are washed with ice-cold phosphate-buffered saline (PBS) once and fixed with prechilled methanol/acetone (7:3) at -20°C for 10 minutes. After they are air-dried, plates are rehydrated with PBS and blocked using 5% nonfat dry milk in PBS-Tween (0.05%) (blocking solution) for 30 minutes at room temperature. Cells are incubated with anti-PAR antibody 10H (1:50) in blocking solution at room temperature for 60 minutes followed by washing with PBS-Tween20 five times, and incubation with goat antimouse fluorescein 5(6)-isothiocyanate (FITC)-coupled antibody (1:50) and 1 μg/mL 40,6-diamidino-2-phenylindole (DAPI) in blocking solution at room temperature for 60 minutes. After washing with PBS-Tween20 5 times, analysis is performed using an fmax Fluorescence Microplate Reader set at the excitation and emission wavelength for FITC or the excitation and emission wavelength for DAPI. PARP activity (FITC signal) is normalized with cell numbers (DAPI). |
| Animal Administration: | A 0.2 cc amount of a 1:10 dilution of tumor brei in 45% Matrigel and 45% Spinner MEM is injected subcutaneously into the flank of female SCID mice on study day 0. Tumors are allowed to grow to the indicated size and then randomized to therapy groups (N=10 mice/group). PARP inhibitor therapy begin on day 14, with cisplatin treatment starting on day 16. At various intervals following tumor inoculation, the individual tumor dimensions are serially measured using calibrated microcalipers, and the tumor volumes are calculated. |
| References: | [1]. Penning TD, et al. Optimization of phenyl-substituted benzimidazole carboxamide poly(ADP-ribose) polymerase inhibitors: identification of (S)-2-(2-fluoro-4-(pyrrolidin-2-yl)phenyl)-1H-benzimidazole-4-carboxamide (A-966492), a highly potent and efficacious |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.