| Cas No.: | 177036-94-1 |
| Chemical Name: | (S)-2-(4,6-Dimethylpyrimidin-2-yloxy)-3-methoxy-3,3-diphenylpropanoic acid |
| Synonyms: | (S)-2-(4,6-Dimethylpyrimidin-2-yloxy)-3-methoxy-3,3-diphenylpropanoic acid;BSF 208075,(+)-(2S)-2-[(4,6-DIMETHYLPYRIMIDIN-2-YL)OXY]-3-METHOXY-3,3-DIPHENYLPROPANOIC ACID;AMBRISENTAN;2-(4,6-Dimethylpyrimidin-2-yl)oxy-3-methoxy-3,3-di(phenyl)propanoic acid;(aS)-a-[(4,6-Dimethyl-2-pyrimidinyl)oxy]--methoxy--phenylbenzenepropanoic Acid;BSF 208075;LU 208075;(+-)-(2S)-2-((4,6-Dimethylpyrimidin-2-yl)oxy)-3-methoxy-3,3-diphenylpropanoic acid,bsf-208075;(2S)-2-(4,6-dimethylpyrimidin-2-yl)oxy-3-methoxy-3,3-diphenylpropanoic acid;(S)-2-((4,6-Dimethylpyrimidin-2-yl)oxy)-3-methoxy-3,3-diphenylpropanoic acid;BSF-208075;Letairis;LU-208075;UNII-HW6NV07QEC;(+)-(2S)-2-[(4,6-dimethylpyrimidin-2-yl)oxy]-3-methoxy-3,3-diphenylpropanoic acid;(S)-2-[(4,6-Dimethylpyrimidin-2-yl)oxy]-3-methoxy-3,3-diphenylpropionic Acid;Ambrisentan Tablets;(R,S)-AMbrisentan-d5;LU-208075,BSF-208075;ambrisentan,CID 6918493;AMbrisentan (BSF 208075) |
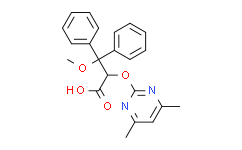
| SMILES: | O=C([C@H](C(OC)(C1=CC=CC=C1)C2=CC=CC=C2)OC3=NC(C)=CC(C)=N3)O |
| Formula: | C22H22N2O4 |
| M.Wt: | 378.42108 |
| Purity: | >99% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Ambrisentan is a selective ET type A receptor (ETAR) antagonist. |
| In Vivo: | In the Ambrisentan group, hepatic hydroxyproline content is significantly lower than in the control group (18.0 μg/g±6.1 μg/g vs 33.9 μg/g±13.5 μg/g liver, respectively, P=0.014). Hepatic fibrosis estimated by Sirius red staining and areas positive for α-smooth muscle actin, indicative of activated hepatic stellate cells, are also significantly lower in the Ambrisentan group (0.46%±0.18% vs 1.11%±0.28%, respectively, P=0.0003; and 0.12%±0.08% vs 0.25%±0.11%, respectively, P=0.047). Moreover, hepatic RNA expression levels of procollagen-1 and tissue inhibitor of metalloproteinase-1 (TIMP-1) are significantly lower by 60% and 45%, respectively, in the Ambrisentan group. Inflammation, steatosis, and endothelin-related mRNA expression in the liver are not significantly different between the groups. Ambrisentan attenuates the progression of hepatic fibrosis by inhibiting hepatic stellate cell activation and reducing procollagen-1 and TIMP-1 gene expression. Ambrisentan did not affect inflammation or steatosis[1]. |
| In Vitro: | Ambrisentan is an endothelin type A receptor antagonist[1]. Ambrisentan induces Nrf2 activation. Endothelial permeability increased in BMEC monolayers at 24 h of hypoxia exposure and compared to vehicle control, Ambrisentan attenuates hypoxia-induced BMEC leak. These results are reversed when prior to treatment BMEC are transfected with siRNA against Nrf2[2]. |
| Cell Assay: | Unless otherwise stated, for each BMEC experiment cells are randomly divided into 4 groups: (1) normoxia vehicle control (Nx-CTRL); (2) normoxia-treated; (3) hypoxia (24 h) control (Hx-CTRL) and (4) hypoxia (24 h) treated. As previously described, Nrf2 activators are added 24 h prior to any hypoxic exposures. Cell treatments are; Protandim (100 μg/mL), methazolamide (125 μg/mL, nifedipine (7 μg/mL) or Ambrisentan (40 μg/mL). In addition, some cells are treated with Nrf2 siRNA. In these experiments, siRNA is added 24 h prior to drug treatments. The rationale for 24 h hypoxia exposure for BMEC is to ensure that cells remained transfected with siRNA for the pre-treatment of drugs (24 h in normoxia) and during the 24 h hypoxia exposure. Data is collected from at least three separate cell culture preparations on three separate days (n=9)[2]. |
| Animal Administration: | Unless otherwise stated, for each BMEC experiment cells are randomly divided into 4 groups: (1) normoxia vehicle control (Nx-CTRL); (2) normoxia-treated; (3) hypoxia (24 h) control (Hx-CTRL) and (4) hypoxia (24 h) treated. As previously described, Nrf2 activators are added 24 h prior to any hypoxic exposures. Cell treatments are; Protandim (100 μg/mL), methazolamide (125 μg/mL, nifedipine (7 μg/mL) or Ambrisentan (40 μg/mL). In addition, some cells are treated with Nrf2 siRNA. In these experiments, siRNA is added 24 h prior to drug treatments. The rationale for 24 h hypoxia exposure for BMEC is to ensure that cells remained transfected with siRNA for the pre-treatment of drugs (24 h in normoxia) and during the 24 h hypoxia exposure. Data is collected from at least three separate cell culture preparations on three separate days (n=9)[2]. |
| References: | Unless otherwise stated, for each BMEC experiment cells are randomly divided into 4 groups: (1) normoxia vehicle control (Nx-CTRL); (2) normoxia-treated; (3) hypoxia (24 h) control (Hx-CTRL) and (4) hypoxia (24 h) treated. As previously described, Nrf2 activators are added 24 h prior to any hypoxic exposures. Cell treatments are; Protandim (100 μg/mL), methazolamide (125 μg/mL, nifedipine (7 μg/mL) or Ambrisentan (40 μg/mL). In addition, some cells are treated with Nrf2 siRNA. In these experiments, siRNA is added 24 h prior to drug treatments. The rationale for 24 h hypoxia exposure for BMEC is to ensure that cells remained transfected with siRNA for the pre-treatment of drugs (24 h in normoxia) and during the 24 h hypoxia exposure. Data is collected from at least three separate cell culture preparations on three separate days (n=9)[2]. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.