| DC31000 |
LP-01
|
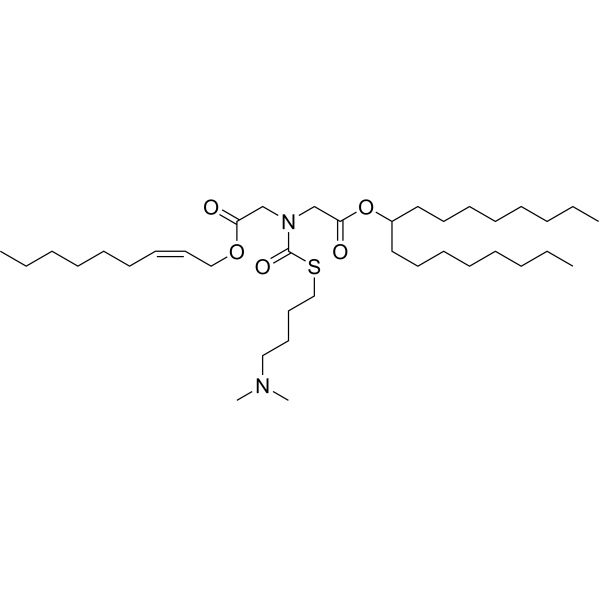
LP-01 is an ionizable cationic amino lipid (pKa = ~6.1). It has been used in the generation of lipid nanoparticles (LNPs). LNPs containing LP-01 and encapsulating both Cas9 mRNA and modified single-guide RNA (sgRNA) for the transport protein transthyretin (Ttr) induce gene editing in liver cells in mice in a dose-dependent manner resulting in reduced serum Ttr levels for at least 12 months. |
| DC57046 |
ATX-126(ATX-0126, lipid 10p)
|
ATX-126(ATX-0126, 10p) is an ionizable cationic lipid (pKa = 6.38).It has been used in the generation of lipid nanoparticles (LNPs) for the delivery of siRNA. Intravenous administration of LNPs containing ATX-126(ATX-0126, 10p) and encapsulating Factor VII siRNA decrease Factor VII blood levels in mice. |
| DC42537 |
ALC-0315
|
ALC-0315 is an ionisable aminolipid that used for mRNA compaction and aids mRNA cellular delivery. ALC-0315 can be used to form lipid nanoparticle (LNP) delivery vehicles. |
| DC52025 |
SM-102
|
SM-102 is an ionizable amino lipid that has been used in combination with other lipids in the formation of lipid nanoparticles.Administration of luciferase mRNA in SM-102-containing lipid nanoparticles induces hepatic luciferase expression in mice. Formulations containing SM-102 have been used in the development of lipid nanoparticles for delivery of mRNA-based vaccines. |
| DC31024 |
SM-86
|
SM86 is a cationic, ionizable lipid developed by Moderna as a core component of its lipid nanoparticle (LNP) platform for mRNA therapeutic delivery.SM-086 is structurally optimized and analogous to SM-102 (used in Moderna’s COVID-19 vaccines), with modifications aimed at enhancing mRNA delivery efficiency and safety.SM-86 serves as the primary cationic lipid in three investigational mRNA therapies targeting rare metabolic disorders:mRNA-3927: Restores propionyl-CoA carboxylase activity in propionic acidemia (PA).
mRNA-3705: Delivers methylmalonyl-CoA mutase mRNA for methylmalonic acidemia (MMA).
mRNA-3210: Provides phenylalanine hydroxylase mRNA to treat phenylketonuria (PKU). |
| DC57100 |
Acuitas Lipid A9
|
Lipid A9 is an ionizable cationic lipid (pKa = 6.27) that has been used in the generation of lipid nanoparticles (LNPs) for the delivery of mRNA and siRNA in vivo. LNPs containing lipid A9 and encapsulating non-stimulatory siRNA increase plasma levels of chemokine (C-C motif) ligand 2 (CCL2), indicating activation of the innate immune response, and decrease body weight in mice. |
| DC60478 |
ALC-0366
|
ALC 0366 is an ionizable cationic lipid (pKa = 6.25) from Biontech,which is derived from ALC-0315. ALC0366 has been used as a key component of LNP to deliver BNT142, a lipid nanoparticle (LNP)-formulated RNA (RNA-LNP) encoding a T cell-engaging bispecific antibody that monovalently binds the T cell marker CD3 and bivalently binds claudin 6 (CLDN6), an oncofetal antigen that is absent from normal adult tissue but expressed on various solid tumors. |
| DC59217 |
Arcturus lipid 2(ATX-0114)
|
Lipid 2,2(8,8) 4C CH3 is an ionizable cationic lipid (pKa = 6.69).1 It has been used in the generation of lipid nanoparticles (LNPs) for the delivery of siRNA in vivo. LNPs containing lipid 2,2(8,8) 4C CH3 and encapsulating siRNA targeting Factor VII decrease plasma Factor VII protein levels by 90% in mice. |
| DC59126 |
Genevant CL1 (lipid 10)
|
Genevant CL1 (lipid 10) is a novel ionizable lipid for rna delivery.Lipid 10 rapidly accumulated in the liver within the first hour of dosing (reflecting LNP uptake), but levels then steadily declined over the ensuing 2 weeks period, similar to MC3.Lipid 10 afforded more than double the expression of either approved lipid. We also observed high splenic expression for ALC-0315, which correlated with higher MCP-1 levels.Animals received a single 5 µg IM dose of LNP encapsulating firefly luciferase (fLuc) mRNA. Whole body imaging was performed 6 h later and expression at the injection site quantified. Lipid 10, ALC-0315, and SM-102 showed similar expression at the injection site, all greater than the older generation benchmarks lipids (DLinDMA, KC2, MC3). Lipid 10 and ALC-0315 also showed high expression in the liver, while SM-102 was less, and more similar to MC3.Lipid 10-based LNP reported similar anti-HA IgG titers to MC3 and ALC-0315 (Comirnaty) LNP, and higher than the SM-102 (SpikeVax) LNP composition. MCP-1 levels were generally similar, although the ALC-0315 composition had a significantly higher response at the 5 µg dose. All formulations reported good stability when stored frozen at −80 °C or at 2–8 °C for 1 month. |
| DC10800 |
DLin-MC3-DMA
|
D-Lin-MC3-DMA(MC3) is the most potent cationic lipid that has been synthesized for Lipid nanoparticles (LNPs) to deliver the siRNA. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.