| Cas No.: | 1146699-66-2 |
| Chemical Name: | Avagacestat |
| Synonyms: | (R)-2-(4-Chloro-N-(2-fluoro-4-(1,2,4-oxadiazol-3-yl)benzyl)phenylsulfonamido)-5,5,5-trifluoropentanamide;BMS-708163;Avagacestat (BMS-708163);(2R)-2-[(4-chlorophenyl)sulfonyl-[[2-fluoro-4-(1,2,4-oxadiazol-3-yl)phenyl]methyl]amino]-5,5,5-trifluoropentanamide;(R)-2-(4-Chloro-N-(2-fluoro-4-(1,2,4-oxadiazol-3-yl)benzyl)-phenylsulfonamido)-5,5,5-trifluoropentanamide;(R)-2-[[4-chlorophenyl)sulfonyl][[2-fluoro-4-(1,2,4-oxadiazol-3-yl)phenyl]methyl]amino]-5,5,5-trifluoropentanamide;Avagacestat;BMS-708163 (avagacestat);Pentanamide, 2-[[(4-chlorophenyl)sulfonyl][[2-fluoro-4-(1,2,4-oxadiazol-3-yl)phenyl]methyl]amino]-5,5,5-trifluoro-, (2R)-;BMS708163;(2R)-2-[N-[(4-Chlorophenyl)sulfonyl]-N-[[2-fluoro-4-(1,2,4-oxadiazol-3-yl)phenyl]methyl]amino]-5,5,5-trifluoropentanamide;(2R)-2-[N-[(4-Chlorophenyl)sulfonyl]-N-[2-fluoro-4-(1,2,4-oxadiazol-3-yl)benzyl]amino]-5,5,5-trifluoropentanamide;BMS 708163;TQ44WWY45Q;(2R)-2-[(4-Chlorophenyl)sulfonyl-[[2-fluoro-4-(1,2,4-oxadiazol-3-yl)phenyl]methyl]amino]-5,5,5-trifluoro-pentanamide;(2R)-5,5,5-trifluoro-2-(N-;(2R)-5,5,5-trifluoro-2-(N-{[2-fluoro-4-(1,2,4-oxadiazol-3-yl)phenyl]methyl}4-chlorobenzenesulfonamido)pentanamide;(2R)-2-[(4-Chlorophenyl)sulfo |
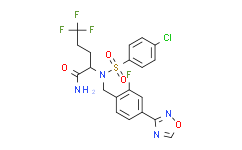
| SMILES: | ClC1C([H])=C([H])C(=C([H])C=1[H])S(N(C([H])([H])C1C([H])=C([H])C(C2=NOC([H])=N2)=C([H])C=1F)[C@@]([H])(C(N([H])[H])=O)C([H])([H])C([H])([H])C(F)(F)F)(=O)=O |
| Formula: | C20H17ClF4N4O4S |
| M.Wt: | 520.885 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | BMS-708163 is a potent inhibitor of γ-secretase, with IC50s of 0.27 nM and 0.30 nM for Aβ42 and Aβ40 inhibition; BMS-708163 also inhibits NICD (Notch IntraCellular Domain) with IC50 of 0.84 nM and shows weak inhibition of CYP2C19, with IC50 of 20 μM. |
| In Vivo: | BMS-708163 significantly reduces both plasma and brain Aβ40 levels relative to control at 10 and 100 mg/kg for the entire dosing interval, demonstrates significant Aβ40 lowering for 8 h after an oral dose of 1 mg/kg, and significantly lowers CSF Aβ40 levels in rats, when measured 5 h after single oral doses ranging from 3 to 100 mg/kg[1]. BMS-708163 (10 mg/kg) monotherapy has a minor inhibitory effect on PC9/AB2 tumor growth compared with gefitinib alone. BMS-708163 monotherapy results in a slight increase in caspase 3 expression as well as a mild decrease in Ki-67 expression in vivo. In the xenograft lung cancer samples treated with BMS-708163 plus gefitinib, there are a marked increase in caspase 3 expression and a reduction in Ki-67 staining[3]. |
| In Vitro: | IC50: 0.27 nM (γ-secretase, Aβ42), 0.30 nM (γ-secretase, Aβ40), 20 μM (CYP2C19)[1], 0.84 nM (NICD)[2] |