| Cas No.: | 501437-28-1 |
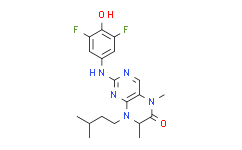
| Chemical Name: | 2-((3,5-Difluoro-4-hydroxyphenyl)amino)-8-isopentyl-5,7-dimethyl-7,8-dihydropteridin-6(5H)-one |
| Synonyms: | 2-((3,5-Difluoro-4-hydroxyphenyl)amino)-8-isopentyl-5,7-dimethyl-7,8-dihydropteridin-6(5H)-one;BI-D1870;2-(3,5-difluoro-4-hydroxyanilino)-5,7-dimethyl-8-(3-methylbutyl)-7H-pteridin-6-one;2-[(3,5-DIFLUORO-4-HYDROXYPHENYL)AMINO]-7,8-DIHYDRO-5,7-DIMETHYL-8-(3-METHYLBUTYL)-6(5H)-PTERIDINONE;HMS3244C11;C19H23F2N5O2;2-(3,5-difluoro-4-hydroxyphenylamino)-8-isopentyl-5,7-dimethyl-7,8-dihydropteridin-6(5H)-one;GTPL8038;BDBM25017;SYN1020;BID |
| SMILES: | FC1C(=C(C([H])=C(C=1[H])N([H])C1=NC([H])=C2C(=N1)N(C([H])([H])C([H])([H])C([H])(C([H])([H])[H])C([H])([H])[H])C([H])(C([H])([H])[H])C(N2C([H])([H])[H])=O)F)O[H] |
| Formula: | C19H23F2N5O2 |
| M.Wt: | 391.415 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | BI-D1870 is an ATP-competitive inhibitor of RSK isoforms, with IC50s of 31 nM/24 nM/18 nM/15 nM for RSK1/SK2/SK3/SK4, respectively. |
| In Vivo: | BI-D1870 (0.5 mg/kg)-injected experimental autoimmune encephalomyelitis (EAE) mice exhibits a delayed neural deficit without obvious weight loss. Histopathological analyses shows inflammatory cell infiltration and demyelination in the spinal cord in control mice, but not in BI-D1870-treated mice. BI-D1870 protects against the infiltration of TH1 or TH17 cells into the CNS[3]. |
| In Vitro: | BI-D1870 inhibits a mutant of RSK2 lacking the C-terminal kinase catalytic domain (RSK21-389:S381E) with an IC50 of approx. 30 nM. BI-D1870 inhibits RSK1 and RSK2 with IC50 values of 10 nM and 20 nM respectively, when the kinase assays are performed with 100 μM ATP. When the assays are performed at a 10-fold lower ATP concentration, the IC50 of BI-D1870 is reduced to 5 nM for RSK1 and 10 nM for RSK2. BI-D1870 inhibits PLK1 with an IC50 of 100 nM, whilst the IC50 values for Aurora B, DYRK1a, CDK2-A, Lck, CK1 and GSK3β are 10- to 100-fold higher than that of the RSK isoforms. BI-D1870 (10 μM) inhibits the PMA-induced phosphorylation of GSK3α and GSK3β in HEK-293 cells. In HEK-293 cells, BI-D1870 inhibits the EGF-induced phosphorylation of LKB1 at Ser431 with an IC50 of approx. 1 μM. Furthermore, BI-D1870 does not affect the activation of ERK1/ERK2 and MSK1, nor does it inhibit the phosphorylation of CREB[1]. BI-D1870 is a potent RSK family kinase inhibitor (Kds: 10-100 nM), and also interact with BRD4(1) and PLK family, with Kds of 3.5 μM and appr 10 nM[2]. BI-D1870 (10 μM) strongly induces p70S6K activation in serum-starved LN-229 cells, and alao stimulates the phosphorylation of rpS6 and p70S6K in LN-18 cells. BI-D1870 (1 μM) potently inhibits rpS6 phosphorylation, and inhibits PMA-induced rpS6 phosphorylation at concentrations higher than 1 μM[4]. BI-D1870 (1-5 μM) induces a dose- and time-dependent inhibition of cell proliferation in all cell types. BI-D1870 (1-3 μM) induces apoptosis in SCC4 cells and HSC-3 cells. BI-D1870 (0-5) modulates cell survival signaling pathways including Akt and p38 MAPK dose-dependently[5]. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.