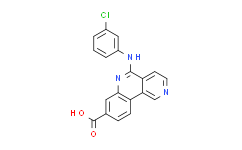
| Cas No.: | 1009820-21-6 |
| Chemical Name: | CX4945 (Silmitasertib) |
| Synonyms: | 5-((3-Chlorophenyl)amino)benzo[c][2,6]naphthyridine-8-carboxylic acid;5-((3-Chlorophenyl)amino)benzo-[c][2,6]naphthyridine-8-carboxylic acid;5-(3-chlorophenylamino)benzo[c][2,6]naphthyridine-8-carboxylic acid;5-[(3-chlorophenyl)amino]-Benzo[c]-2,6-naphthyridine-8-carboxylic acid;CX-4945;CX-4945 (CX 4945, CX4945, Silmitasertib);CX-4945 (Silmitasertib);CX-4945 Silmitasertib;CX-4945;Silmitasertib;5-(3-chloroanilino)benzo[c][2,6]naphthyridine-8-carboxylic acid;CX 4945;5-[(3-Chlorophenyl)amino]benzo[c]-2,6-naphthyridine-8-carboxylic acid;CX-4945, CX4945;Silmitasertib(CX-4945);5-[(3-Chlorophenyl)amino]benzo[c]-2,6-naphthyridine-8-carboxylic acid CX-4945 (Silmitasertib);CX4945;C6RWP0N0L2;5-[(3-Chlorophenyl)amino]benzo[c][2,6]naphthyridine-8-Carboxylic Acid;5-((3-Chl |
| SMILES: | ClC1=C([H])C([H])=C([H])C(=C1[H])N([H])C1C2C([H])=C([H])N=C([H])C=2C2C([H])=C([H])C(C(=O)O[H])=C([H])C=2N=1 |
| Formula: | C19H12ClN3O2 |
| M.Wt: | 349.77 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | CX-4945 is a potent, selective and oral casein kinase 2 (CK2) inhibitor with a Ki of 0.38 nM. |
| In Vivo: | CX-4945 (25 or 75 mg/kg, p.o.) is well tolerated and demonstrated robust antitumor activity with concomitant reductions of the mechanism-based biomarker phospho-p21 (T145) in murine xenograft models[1]. |
| In Vitro: | CX-4945 causes cell-cycle arrest and selectively induces apoptosis in cancer cells relative to normal cells, attenuates PI3K/Akt signalingand, and the antiproliferative activity of CX-4945 is correlated with expression levels of the CK2α catalytic subunit, Attenuation of PI3K/Akt signaling[1]. CX-4945 with bortezomib treatment prevents leukemic cells from engaging a functional UPR in order to buffer the bortezomib-mediated proteotoxic stress in ER lumen, and decreases pro-survival ER chaperon BIP/Grp78 expression[2]. CX-4945 induces cytotoxicity and apoptosis, and exerts anti-proliferative effects in hematological tumors by downregulating CK2 expression and suppressing activation of CK2-mediated PI3K/Akt/mTOR signaling pathways[3]. |