| Cas No.: | 167221-71-8 |
| Chemical Name: | Clevidipine |
| Synonyms: | Methyl (1-oxobutoxy)methyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dime thyl-3,5-pyridinedicarboxylate;Clevidipine butyrate;4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-Pyridinedicarboxylic acid methyl (1-oxobutoxy)methyl ester;Cleviprex;5-O-(butanoyloxymethyl) 3-O-methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate;Clevidipine;Clevelox;Clevidipine,Cleviprex;H 324;rac-Clevidipine;3-[(Butyryloxy)methyl] 5-Methyl 4-(2,3-Dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate;4-(2,3-Dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylic Aicd 3-[(Butyryloxy)methyl] 5-Methyl Ester;Cleviprex (Clevidipine);Butyric acid Clevidipine;Cleviprex Clevidipine butyrate;3-((Butyryloxy)methyl) 5-methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-di;H 324/38;Clevidipine Butyrate/Cleviprex;Methyl (1-oxobutoxy)methyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate;METHYL 5-{[(BUTANOYLOXY)METHOXY]CARBONYL}-4-(2,3-DICHLOROPHENYL)-2,6-DIMETHYL-1,4-DIHYDROPYRIDINE-3 |
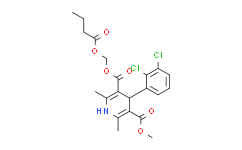
| SMILES: | ClC1C(=C([H])C([H])=C([H])C=1C1([H])C(C(=O)OC([H])([H])[H])=C(C([H])([H])[H])N([H])C(C([H])([H])[H])=C1C(=O)OC([H])([H])OC(C([H])([H])C([H])([H])C([H])([H])[H])=O)Cl |
| Formula: | C21H23Cl2NO6 |
| M.Wt: | 456.3164 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Clevidipine is a short-acting dihydropyridine calcium channel antagonist (IC50= 7.1 nM, V(H) = -40 mV ) under development for treatment of perioperative hypertension. |
| References: | [1]. Yi X, Vivien B, Lynch C 3rd. Clevidipine blockade of L-type Ca2+ currents: steady-state and kinetic electrophysiological studies in guinea pigventricular myocytes. [2]. Huraux C, Makita T, Szlam F, The vasodilator effects of clevidipine on human internal mammary artery. Anesth Analg. 1997 Nov;85(5):1000-4. [3]. Ericsson H, et al. In vitro hydrolysis rate and protein binding of clevidipine, a new ultrashort-acting calcium antagonist metabolised by esterases, in different animal species and man. Eur J Pharm Sci. 1999 Apr;8(1):29-37. [4]. Ericsson H, et al. Pharmacokinetics of new calcium channel antagonist clevidipine in the rat, rabbit, and dog and pharmacokinetic/pharmacodynamic relationship in anesthetized dogs. Drug Metab Dispos. 1999 May;27(5):558-64. [5]. Schwieler JH, et al. Circulatory effects and pharmacology of clevidipine, a novel ultra short acting and vascular selective calcium antagonist, in hypertensive humans. J Cardiovasc Pharmacol. 1999 Aug;34(2):268-74. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.