| Cas No.: | 869998-49-2 |
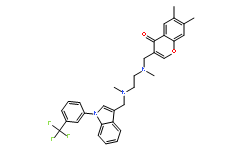
| Chemical Name: | 6,7-dimethyl-3-[[methyl-[2-[methyl-[[1-[3-(trifluoromethyl)phenyl]indol-3-yl]methyl]amino]ethyl]amino]methyl]chromen-4-one |
| Synonyms: | 6,7-dimethyl-3-[[methyl-[2-[methyl-[[1-[3-(trifluoromethyl)phenyl]indol-3-yl]methyl]amino]ethyl]amino]methyl]chromen-4-one;CHEMBL255489;AC1NS1JP;SureCN1270919;MolPort-009-019-121;AG-L-65178;6,7-DIMETHYL-3-[(METHYL{2-[METHYL({1-[3-(TRIFLUOROMETHYL)PHENYL]-1H-INDOL-3-YL}METHYL)AMINO]ETHYL}AMINO)METHYL]-4H-CHROMEN-4-ONE;SPD 304;SPD-304 |
| SMILES: | CN(CCN(C)CC1C2C(=CC=CC=2)N(C=1)C3C=C(C=CC=3)C(F)(F)F)CC4C(=O)C5C(OC=4)=CC(C)=C(C)C=5 |
| Formula: | C32H32F3N3O2 |
| M.Wt: | 547.610598564148 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | SPD304 is a selective inhibitor of tumor necrosis factor α (TNFα) and promotes dissociation of TNF trimers and therefore blocks the interaction of TNF and its receptor, with an IC50 of 22 µM for inhibiting in vitro TNF receptor 1 (TNFR1) binding to TNF-α[1][2]. SPD304 cannot be used in vivo due to its high toxicity[3]. |
| Target: | IC50: 22 µM (TNFα)[1]. |
| In Vitro: | SPD304 (2 μM) significantly rescues the survivability of aHSCs, reduces the production of lipid hydroxides, and increased intracellular GSH. The co-treatment of GA (75 μM) and SPD304 (2 μM), down-regulate TRADD almost 2-fold (w/o inhibitor vs. w/ inhibitor) and p−RIP3 1.4−fold compared to GA alone, and promotes caspase 8 activation[4]. |
| References: | [1]. Molly M. He, et al. Small-Molecule Inhibition of TNF-α. Science 11 Nov 2005. [2]. Alexiou P, et al. Rationally designed less toxic SPD-304 analogs and preliminary evaluation of their TNF inhibitory effects. Arch Pharm (Weinheim). 2014 Nov;347(11):798-805. [3]. Mouhsine H, et al. Identification of an in vivo orally active dual-binding protein-protein interaction inhibitor targeting TNFα through combined in silico/in vitro/in vivo screening. Sci Rep. 2017 Jun 13;7(1):3424. [4]. Gallic acid induces necroptosis via TNF-α signaling pathway in activated hepatic stellate cells. Chang YJ, et al. PLoS One. 2015 Mar 27;10(3):e0120713. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.