| Cas No.: | 119431-25-3 |
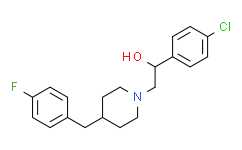
| Chemical Name: | Eliprodil |
| Synonyms: | 1-(4-Chlorophenyl)-2-(4-(4-fluorobenzyl)piperidin-1-yl)ethanol;Eliprodil;1-Piperidineethanol, a-(4-chlorophenyl)-4-[(4-fluorophenyl)methyl]-;Eliprodil,α-(4-Chlorophenyl)-4-[(4-fluorophenyl)methyl]-1-piperidineethanol;SL-820715;Α-(4-CHLOROPHENYL)-4-[(4-FLUOROPHENYL)METHYL]-1-PIPERIDINEETHANOL;SL 820715;alpha-(4-Chlorophenyl)-4-[(4-fluorophenyl)methyl]-1-piperidineethanol;1-(4-broMophenyl)-2-(4-(4-fluorobenzyl)piperidin-1-yl)ethanol;(+-)-alpha-(p-Chlorophenyl)-4-(p-fluorobenzyl)-1-piperidineethanol;SL 820715, α-(4-Chlorophenyl)-4-[(4-fluorophenyl)methyl]-1-piperidineethanol;Eliprodil [INN];C20H23ClFNO;1-Piperidineethanol, alpha-(4-chlorophenyl)-4-(4-fluorophenyl)-, (+-)-;DSSTox_RID_81089;DSSTox_CID_25744;DSSTox_GSID_45744;1-(4-Chloro-phenyl)-2-[4-(4-fluoro-benzyl)-piperidin-1-yl]-ethanol;(+-)-alpha-(p-Chlorophenyl)-4-(p-fluorobenzyl)-1-piperi |
| SMILES: | ClC1C([H])=C([H])C(=C([H])C=1[H])C([H])(C([H])([H])N1C([H])([H])C([H])([H])C([H])(C([H])([H])C2C([H])=C([H])C(=C([H])C=2[H])F)C([H])([H])C1([H])[H])O[H] |
| Formula: | C20H23ClFNO |
| M.Wt: | 347.8541 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Eliprodil(SL-820715) is a non-competitive NR2B-NMDA receptor antagonist(IC50=1 uM), less potent for NR2A- and NR2C-containing receptors(IC50> 100 uM).IC50 value:Target: NR2B-NMDA antagonistHuman N-type Ca2+ channel currents were inhibited by ifenprodil and eliprodil with IC50 values of 50 microM and 10 microM respectively whereas P-type Ca2+ channel currents were inhibited reversibly by ifenprodil and eliprodil with approximate IC50 values of 60 microM and 9 microM respectively. eliprodil (1 microm) produced a moderate reverse rate-dependent prolongation of the action potential duration (7.4+/-1.5, 8.9+/-2.1 and 9.9+/-1.8% at cycle lengths of 300, 1000 and 5000 ms, respectively; n=9). |
| References: | [1]. Bath CP, et al. The effects of ifenprodil and eliprodil on voltage-dependent Ca2+ channels and in gerbil global cerebral ischaemia. Eur J Pharmacol. 1996 Mar 28;299(1-3):103-12. [2]. Lengyel C, et al. Effect of a neuroprotective drug, eliprodil on cardiac repolarisation: importance of the decreased repolarisation reserve in the development of proarrhythmic risk. Br J Pharmacol. 2004 Sep;143(1):152-8. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.