| Cas No.: | 134381-21-8 |
| Chemical Name: | L-Threoninamide,N-acetyl-N-methyl-L-isoleucyl-L-isoleucyl-N-[(1S)-3-methyl-1-[[(2R)-2-methyl-2-oxiranyl]carbonyl]butyl]- |
| Synonyms: | L-Threoninamide,N-acetyl-N-methyl-L-isoleucyl-L-isoleucyl-N-[(1S)-3-methyl-1-[[(2R)-2-methyl-2-oxiranyl]carbonyl]butyl]-;(2S,3S)-2-[[(2S,3S)-2-[acetyl(methyl)amino]-3-methylpentanoyl]amino]-N-[(2S,3R)-3-hydroxy-2-[[(2S)-4-methyl-1-[(2R)-2-methyloxiran-2-yl]-1-oxopentan-2-yl]amino]butanoyl]-3-methylpentanamide;BU-4061T;Epoxomicin;Epoxomicin (BU 4061T, BU-4061T);Epoxomicin (BU-4061T);S7038,BU-4061T;N-Acetyl-N-methyl-L-isoleucyl-L-isoleucyl-N-[(1S)-3-methyl-1-[[(2R)-2-methyl-2-oxiranyl]carbonyl]butyl]-L-threoninamide;BU 4061T |
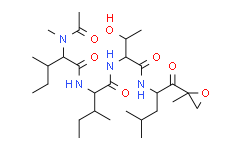
| SMILES: | CCC(C)C(NC(=O)C(C(C)CC)N(C)C(C)=O)C(=O)NC(C(C)O)C(=O)NC(CC(C)C)C(=O)C1(C)OC1 |
| Formula: | C28H50N4O7 |
| M.Wt: | 554.7192 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Epoxomicin is a cell-permeable and irreversible proteasome inhibitor, primarily the chymotrypsin-like activity. |
| References: | [1]. Kim KB, et al. Proteasome inhibition by the natural products epoxomicin and dihydroeponemycin: insights into specificity and potency. Bioorg Med Chem Lett. 1999 Dec 6;9(23):3335-40. [2]. Hanada M, et al. Epoxomicin, a new antitumor agent of microbial origin. J Antibiot (Tokyo). 1992 Nov;45(11):1746-52. [3]. Garrett IR, et al. Selective inhibitors of the osteoblast proteasome stimulate bone formation in vivo and in vitro. J Clin Invest. 2003 Jun;111(11):1771-82. [4]. McNaught KS, et al. Systemic exposure to proteasome inhibitors causes a progressive model of Parkinson's disease. Ann Neurol. 2004 Jul;56(1):149-62. [5]. Rideout HJ, et al. Dopaminergic neurons in rat ventral midbrain cultures undergo selective apoptosis and form inclusions, but do not up-regulate iHSP70, following proteasomal inhibition. J Neurochem. 2005 Jun;93(5):1304-13. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.