| Cas No.: | 1286739-19-2 |
| Chemical Name: | 6-[2-Chloro-4-(1,3-thiazol-5-yl)phenyl]-8-ethyl-2-[4-(4-methylpiperazin-1-yl)anilino]pyrido[2,3-d]pyrimidin-7-one |
| Synonyms: | FRAX597;6-(2-Chloro-4-thiazol-5-yl-phenyl)-8-ethyl-2-[4-(4-Methyl-piperazin-1-yl)-phenylaMino]-8H-pyrido[2,3-d]pyriMidin-7-one;6-[2-chloro-4-(1,3-thiazol-5-yl)phenyl]-8-ethyl-2-[4-(4-methylpiperazin-1-yl)anilino]pyrido[2,3-d]pyrimidin-7-one,hydrochloride;FRAX-597;6-[2-Chloro-4-(5-thiazolyl)phenyl]-8-ethyl-2-[[4-(4-methyl-1-piperazinyl)phenyl]amino]pyrido[2,3-d]pyrimidin-7(8H)-one;FRAX 597;Pyrido[2,3-d]pyrimidin-7(8H)-one, 6-[2-chloro-4-(5-thiazolyl)phenyl]-8-ethyl-2-[[4-(4-methyl-1-piperazinyl)phenyl]amino]-;GTPL8939;AMX10195;BCP12649;s7271;BDBM50112347;SB19060;AK174921;6-[2-Chloro-4-(5-thiazolyl)phenyl]-8-ethyl-2-[[4-(4-methyl-1-piperazinyl)phenyl]amino]pyrido[2,3-d]pyrimidin-7-(8H)-one;6-[2-chlor;6-[2-Chloro-4-(1,3-thiazol-5-yl)phenyl]-8-ethyl-2-[4-(4-methylpiperazin-1-yl)anilino]pyrido[2,3-d]pyrimidin-7-one |
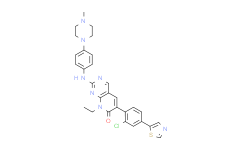
| SMILES: | ClC1C([H])=C(C2=C([H])N=C([H])S2)C([H])=C([H])C=1C1=C([H])C2=C([H])N=C(N=C2N(C([H])([H])C([H])([H])[H])C1=O)N([H])C1C([H])=C([H])C(=C([H])C=1[H])N1C([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])C1([H])[H] |
| Formula: | C29H28ClN7Os |
| M.Wt: | 558.0969 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | FRAX597 is a potent group I p21-activated Kinases (PAKs) inhibitor with IC50 of 8, 13 and 19 nM for PAK1, 2 and 3. |
| In Vivo: | Analysis of the flux reading for the animals in the two cohorts demonstrates a significantly slower tumor growth rate in FRAX597-treated mice compared with control mice. After 14 days of treatment the animals are sacrificed and the tumors excised and weighed. FRAX597-treated cohort shows significantly lower average tumor weight compared with the control cohort (0.55 g versus 1.87 g, p=0.0001)[1]. |
| In Vitro: | FRAX597 is determined to be a potent, ATP-competitive inhibitor of group I PAKs (PAK 1-3), with biochemical IC50 values as follows: PAK1 IC50=8 nM, PAK2 IC50=13 nM, PAK3 IC50=19 nM. The IC50 toward PAK4, a member of group II PAKs is >10 μM. At a concentration of 100 nM FRAX597 displays a significant (>80% inhibition) inhibitory capacity toward YES1 (87%), RET (82%), CSF1R (91%), TEK (87%), PAK1 (82%), and PAK2 (93%). When measured using the Kinase Glo Assay in the presence of 20 nM protein and 1 μM ATP, FRAX597 displayed an IC50 value of 48 nM against wild type PAK1, while IC50 values against the V342F and V342Y PAK1 mutants are higher than 3 μM and 2 μM, respectively[1]. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.