| Cas No.: | 67469-81-2 |
| Chemical Name: | GBR-12935 HCl |
| Synonyms: | Piperazine,1-[2-(diphenylmethoxy)ethyl]-4-(3-phenylpropyl)-, hydrochloride (1:2);1-(2-(BENZHYDRYLOXY)ETHYL)-4-(3-PHENYLPROPYL)PIPERAZINE.2HCL;1-(2-benzhydryloxyethyl)-4-(3-phenylpropyl)piperazine,dihydrochloride;1-(2-DIPHENYLMETHOXYETHYL)-4-(3-PHENYLPROPYL)PIPERAZINE DIHYDROCHLORIDE;GBR 12935 dihydrochloride;{1-[2-(diphenylmethoxy)ethyl]-4-(3-phenyl-propyl)piperazine} dihydrochloride;1-[2-(diphenylmethoxy)ethyl]-4-(3-phenylpropyl)-piperazine;1-[2-(Diphenylmethoxy)ethyl]-4-(3-phenylpropyl)piperazine dihydrochloride;AGN-PC-00IQSG;CHEBI:64091;CTK8G0008;EU-0100560;GBR-12935 dihydrochloride;SureCN339982;GBR 12935 (dihydrochloride);GBR 12935 HCl;1-[2-(diphenylmethoxy)ethyl]-4-(3-phenylpropyl)piperazinediium dichloride;1-[2-(Diphenylmethoxy)ethyl]-4-(3-phenylpropyl)-piperazine dihydrochloride;1-[2-(benzhydryloxy)ethyl]-4-(3-phenylpropyl)piperazine dihydrochloride;SR- |
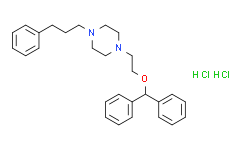
| SMILES: | Cl[H].Cl[H].O(C([H])(C1C([H])=C([H])C([H])=C([H])C=1[H])C1C([H])=C([H])C([H])=C([H])C=1[H])C([H])([H])C([H])([H])N1C([H])([H])C([H])([H])N(C([H])([H])C([H])([H])C([H])([H])C2C([H])=C([H])C([H])=C([H])C=2[H])C([H])([H])C1([H])[H] |
| Formula: | C28H36Cl2N2O |
| M.Wt: | 487.509 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | GBR 12935 2Hcl is a potent, and selective dopamine reuptake inhibitor.IC50 value: Target: dopamine reuptake inhibitorin vitro: The calculated Kd of [3H]GBR-12935 binding to CYP2D6 was 42.2 nM, indicating that GBR-12935 has a high affinity for CYP2D6. The binding of [3H]GBR-12935 to CYP2D6 was decreased partially by substrates or inhibitors of CYP2D isoforms (quinine, quinidine, propranolol, bufuralol, imipramine, and desipramine) [1]. Co-perfusion of 100 microM GBR 12909 or GBR 12935 with either 100 microM sulpiride or raclopride produced a significant reduction in the GBR 12909 or GBR 12935 induced increase in the extracellular levels of dopamine to basal levels. In vitro, GBR 12909 (1-9 nM) dose-dependently inhibited active uptake of [3H]dopamine in homogenates of the nucleus accumbens [2].in vivo: GBR 12935 elevated locomotion to a greater extent in C57BL/6J mice at the maximally active dose of 10 mg/kg. Locomotor stimulation by GBR 12935 remained consistent in both strains with repeated injections. DBA/2J mice became sensitized to cocaine-induced stereotypy with repeated injections. Cocaine induced no stereotypy in C57BL/6J mice on any test day. No stereotypies were induced by GBR 12935 in either strain on any test day [3]. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.