| Synonyms: |
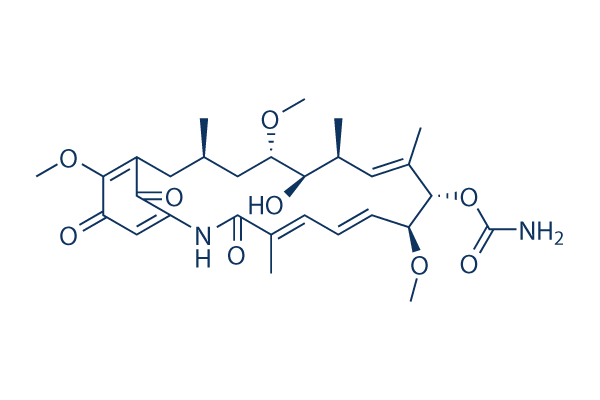
Geldanamycin;GeldanamyCln;Geldanamycin from Streptomyces hygroscopicus;Geldanamycin (Geldanomycin,U-29135, NSC 122750);GeldanaMycin, froM StreptoMyces hygroscopicus var geldanus;GELDANAMYCIN FROM STRE;GELDANAMYCIN,STREPTOMYCES HYGROSCOPICUS;Streptomyces hygroscopicus;[(3R,5R,6S,7R,8E,10R,11R,12Z,14E)-6-Hydroxy-5,11,21-trimethoxy-3,7,9,15-tetramethyl-16,20,22-trioxo-17-azabicyclo[16.3.1]docosa-1(21),8,12,14,18-pentaen-10-yl] carbamate;CCG-208040;HMS1990E15;2-Azabicyclo[16.3.1]docosa-4,6,10,18,21-pentaene-3,20,22-trione, 9-[(aminocarbonyl)oxy]-13-hydroxy-8,14,19-trimethoxy-4,10,12,16-tetramethyl-, [8S-(4E,6Z,8R*,9R*,10E,12R*,13S*,14R*,16S*)]-;SCHEMBL4154;GTPL9829;U-29135;HMS1792E15;GELDANAMYCIN [MI];BRN 1633093;BSPBio_001073;G0334;GMY;NSC 122750;NCGC00163449-02;MFCD00274570;geldanamycin;HMS3403E15;Geldanamycin from Streptomyces hygroscopicus, >=98% (HPLC), powder;SR-05000002266-3;[8S-(4E,6Z,8R*,9R*,10E,12R*,13S*,14R*,16S*)]-9-[(Aminocarbonyl)oxy]-13-hydroxy-8,14,19-trimethoxy-4,10,12,16-tetramethyl-2-azabicyclo[16.3.1]docosa-4,6,10,18,21-pentaene-3,20,22-trione;SCHEMBL13037476;QTQAWLPCGQOSGP-KSRBKZBZSA-N;Geldanamycin, Streptomyces hygroscopicus - CAS 30562-34-6;GDM;[(4E,6Z,8S,9S,10E,12S,13R,14S,16R)-13-hydroxy-8,14,19-trimethoxy-4,10,12,16-tetramethyl-3,20,22-trioxo-2-azabicyclo[16.3.1]docosa-1(21),4,6,10,18-pentaen-9-yl] carbamate;2-azabicyclo[16.3.1]docosa-4,6,10,18,21-pentaene-3,20,22-trione, 9-[(aminocarbonyl)oxy]-13-hydroxy-8,14,19-trimethoxy-4,10,12,16-tetramethyl-, (4E,6Z,8S,9S,10E,12S,13R,14S,16R)-;[(3R,5S,6R,7S,8E,10S,11S,12Z,14E)-6-hydroxy-5,11,21-trimethoxy-3,7,9,15-tetramethyl-16,20,22-trioxo-17-azabicyclo[16.3.1]docosa-1(21),8,12,14,18-pentaen-10-yl] carbamate;HMS1362E15;EI-280;U 29135;(4E,6Z,8S,9S,10E,12S,13R,14S,16R)-13-hydroxy-8,14,19-trimethoxy-4,10,12,16-tetramethyl-3,20,22-trioxo-2-azabicyclo[16.3.1]docosa-1(21),4,6,10,18-pentaen-9-yl carbamate;DB02424;Z2568648438;1yet;[(3S,5S,6R,7S,8E,10R,11S,12Z,14E)-6-Hydroxy-5,11,21-trimethoxy-3,7,9,15-tetramethyl-16,20,22-trioxo-17-azabicyclo[16.3.1]docosa-8,12,14,18,21-pentaen-10-yl] carbamate;BDBM50008059;Geldanamycin (9CI);Z3K3VJ16KU;SR-05000002266;2-Azabicyclo[16.3.1]docosa-4,6,10,18,21-pentaene-3,20,22-trione, 9,13-dihydroxy-8,14,19-trimethoxy-4,10,12,16-tetramethyl-, 9-carbamate (8CI);UNII-Z3K3VJ16KU;IDI1_002122;NSC-122750;CHEBI:91381;BRD-K11528507-001-01-8;NCGC00163449-01;CHEBI:5292;NS00011696;J-018017;AKOS024456552;J-521410;DTXSID7042691;30562-34-6;A1-50253;2-Azabicyclo[16.3.1]docosa-4,10,18,21-pentaene- 3,20,22-trione, 9,13-dihydroxy-8,14,19-trimethoxy- 4,10,12,16-tetramethyl-, 9-carbamate;2-Azabicyclo[16.3.1]docosa-4,10,18,21-pentaene- 3,20,22-trione, 9,13-dihydroxy-8,14,19-trimethoxy- 4,10,12,16-tetramethyl-, 9-carbamate;Antibiotic U 29135;Antibiotic U 29135;NSC122750;NSC122750;CHEMBL182055;CHEMBL182055;Antibiotic (U-29,135);Antibiotic (U-29,135);Geldanamicin;Gel... |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.