| Cas No.: | 59870-68-7 |
| Chemical Name: | Glabridin |
| Synonyms: | Glabridin;4-[(3R)-8,8-Dimethyl-3,4-dihydro-2H-pyrano[6,5-f]chromen-3-yl]benzene-1,3-diol;4-[(3R)-8,8-dimethyl-3,4-dihydro-2H-pyrano[2,3-f]chromen-3-yl]benzene-1,3-diol;GLABRIDIN(RG);GLABRIDIN(RG) PrintBack;1,3-Benzenediol,4-(3,4-dihydro-8,8-dimethyl-2H,8H-benzo(1,2-b:3,4-b')dipyran-3-yl)-,(R);4-(3,4-Dihydro-8,8-dimethyl-2H,8H-benzo(1,2-b:3,4-b')dipyran-3-yl)-1,3-benzenediol;BIDD:ER0172;Bio-0904;Glabridin?;(R)-4-(3,4-Dihydro-8,8-dimethyl-2H,8H-benzo[1,2-b:3,4-b′]dipyran-3-yl)-1,3-benzenediol;4-[(3R)-3,4-Dihydro-8,8-dimethyl-2H,8H-benzo[1,2-b:3,4-b′]dipyran-3-yl]-1,3-benzenediol;[ "" ];HOC5567T41;C20H20O4;1,3-Benzenediol, 4-(3,4-dihydro-8,8-dimethyl-2H,8H-benzo(1,2-b:3,4-b')dipyran-3-yl)-, (R)-;GlaBioidin;glabridine;;MLS000697609;Glabridin, analytical standard;AOB4382;HMS2271G1 |
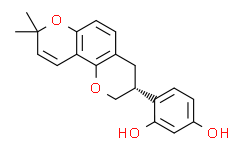
| SMILES: | O1C2C3C([H])=C([H])C(C([H])([H])[H])(C([H])([H])[H])OC=3C([H])=C([H])C=2C([H])([H])[C@]([H])(C2C([H])=C([H])C(=C([H])C=2O[H])O[H])C1([H])[H] |
| Formula: | C20H20O4 |
| M.Wt: | 324.3704 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Glabridin is a natural isoflavan from Glycyrrhiza glabra, binds to and activates PPARγ, with an EC50 of 6115 nM. Glabridin exhibits antioxidant, anti-bacterial, anti-nephritic, anti-diabetic, anti-fungal, antitumor, anti-inflammatory, antiosteoporotic, cardiovascular protective, neuroprotective and radical scavenging activities[1][2]. |
| In Vivo: | Glabridin (50 mg/kg, p.o. once daily) shows potent anti-inflammatory activity, ameliorates the inflammatory alterations induced by Dextran sodium sulphate (DSS) in rats[3]. |
| In Vitro: | Glabridin binds to and activates PPARγ, with an EC50 of 6115 nM[1]. Glabridin (40, 80 μM) inhibits the proliferation of SCC-9 and SAS cell lines in a dose- and time-dependent manner after treatment for 24 and 48 h[2]. Glabridin (0-80 μM) also induces apoptosis, causes Sub-G1 cell cycle arrest in SCC-9 and SAS cell lines[2]. Glabridin (0, 20, 40, and 80 μM) dose-dependently activates caspase-3, −8, and −9 and increases PARP cleavage, significantly phosphorylates ERK1/2, JNK1/2, and p-38 MAPK in SCC-9 cells[2]. |