| Cas No.: | 780757-88-2 |
| Chemical Name: | ICG001 |
| Synonyms: | (6S,9aS)-N-Benzyl-6-(4-hydroxybenzyl)-8-(naphthalen-1-ylmethyl)-4,7-dioxooctahydro-1H-pyrazino[1,2-a]pyrimidine-1-carboxamide;(6S,9AS)-N-BENZYL-6-(4-HYDROXYBENZYL)-8-(NAPHTHALEN-1-YLMETHYL)-4,7-DIOXOOCTAHYDRO-1H-PYRAZINO[1,2-A]PYRIMIDINE-1-CARBO...;2H-PYRAZINO[1,2-A]PYRIMIDINE-1(6H)-CARBOXAMIDE,HEXAHYDRO-6-[(4-HYDROXYPHENYL)METHYL]-8-(1-NAPHTHALENYLMETHYL)-4,7-DIOXO-;2H-PYRAZINO[1,2-A]PYRIMIDINE-1(6H)-CARBOXAMIDE,HEXAHYDRO-6-[(4-HYDROXYPHENYL)METHYL]-8-(1-NAPHTHALENYLMETHYL)-4,7-DIOXO-N-(PHENYLMETHYL)-,(6R,9AR)-REL-;ICG 001;ICG-001;ICG-001 (ICG001, ICG 001);(6R,9aR)-rel-Hexahydro-6-[(4-hydroxyphenyl)methyl]-8-(1-naphthalenylmethyl)-4,7-dioxo-N-(phenylmethyl)-2H-pyrazino[1,2-;(6S,9AS)-6-(4-HYDROXYBENZYL)-N-BENZYL-8-(NAPHTHALEN-1-YLMETHYL)-4,7-DIOXO-HEXAHYDRO-2H-PYRAZINO[1,2-A]PYRIMIDINE-1(6H)-CARBOXAMIDE;(6S,9aS)-N-benzyl-6-(4-hydroxybenzyl)-8-(naphthalen-1-ylMethyl);ICG001;PRI 724;(6s,9as)-n-benzyl-6-(4-hydroxybenzyl)-8-(1-naphthylmethyl)-4,7-di Oxohexahydro-2h-pyrazino[1,2-a]pyrimidine-1(6h)-carboxamide;C33H32N4O4;(6S,9AS)-HEXAHYDRO-6-[(4-HYDROXYPHENYL)METHYL]-8-(1-NAPHTHALENYLMETHYL)-4,7-DIOXO-N-(PHENYLMETHYL)-2H-PYRAZINO[1,2-A]PYRIMIDINE-1(6H)-CARBOXAMIDE;(6S,9aS)-6-(4-hydroxybenzyl)-N-benzy;(6S,9As)-N-benzyl-6-[(4-hydroxyphenyl)methyl]-8-(naphthalen-1-ylmethyl)-4,7-dioxo-3,6,9,9a-tetrahydr |
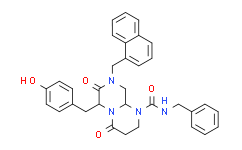
| SMILES: | O=C1[C@]([H])(C([H])([H])C2C([H])=C([H])C(=C([H])C=2[H])O[H])N2C(C([H])([H])C([H])([H])N(C(N([H])C([H])([H])C3C([H])=C([H])C([H])=C([H])C=3[H])=O)[C@@]2([H])C([H])([H])N1C([H])([H])C1=C([H])C([H])=C([H])C2=C([H])C([H])=C([H])C([H])=C12)=O |
| Formula: | C33H32N4O4 |
| M.Wt: | 548.643 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | ICG-001 is an inhibitor of β-catenin/TCF mediated transcription. It works by specifically binding to cyclic AMP response element-binding protein with an IC50 of 3 μM. |
| In Vivo: | ICG-001 (5 mg/kg per day) significantly inhibits beta-catenin signaling and attenuates bleomycin-induced lung fibrosis in mice, while concurrently preserving the epithelium[2]. Administration of a water-soluble analog of ICG-001 for 9 weeks reduces the formation of colon and small intestinal polyps by 42% as effectively as the nonsteroidal antiinflammatory agent Sulindac, which has consistently demonstrated efficacy in this model. ICG-001 (150 mg/kg, i.v.) demonstrates a dramatic reduction in tumor volume over the 19-day course of treatment, with no mortality or weight loss in the SW620 nude mouse xenograft model of tumor regression[3]. |
| In Vitro: | ICG-001 (5μM) inhibits leptin-induced EMT, invasion and tumorsphere formation in MCF7 cells[1]. ICG-001 can phenotypically rescue normal nerve growth factor (NGF)-induced neuronal differentiation and neurite outgrowth in the presenilin-1 mutant cells, emphasizing the importance of the TCF/β-catenin signaling pathway on neurite outgrowth and neuronal differentiation[2]. ICG-001 (25μM) treatment reduces the steady-state levels of Survivin and Cyclin D1 RNA and protein in SW480 cells, both of which can be up-regulated by β-catenin. ICG-001 selectively induces apoptosis in transformed cells but not in normal colon cells, and reduces in vitro growth of colon carcinoma cells[3]. |
| Cell Assay: | To evaluate effects of ICG-001 on α-SMA and collagen type 1 expression, RLE-6TN cells are treated with TGF-β1 (0.25 ng/mL) in the presence or absence of ICG-001 (5.0 μM). After 24 h, cells are harvested and mRNA isolated for analysis by qPCR. RNA is reverse-transcribed using SuperScript reverse transcriptase. Quantitative PCR is performed with SYBR-Green PCR using Real-Time PCR System HT7900. The amplification protocol is set as follows: 95°C denaturation for 10 min followed by 40 cycles of 15-s denaturation at 95°C, 1 min of annealing/extension, and data collection at 60°C. |
| Animal Administration: | Seven-week-old male C57BL/6J-ApcMin/+and WT C57BL/6J mice are treated orally for 9 weeks with ICG-001a (300 mg/kg per day) or vehicle (1% carboxymethylcellulose), once daily, six times per week. Sulindac is administered in drinking water (160 ppm, dissolved in 8 mM Na2PO4 buffer, pH 7.6). At 16 weeks, the polyp number is counted manually by using a dissecting microscope. |
| References: | [1]. Yan D, et al, Leptin-induced epithelial-mesenchymal transition in breast cancer cells requires β-catenin activation via Akt/GSK3- and MTA1/Wnt1 protein-dependent pathways. J Biol Chem, 2012, 287(11), 8598-8612. [2]. Henderson WR Jr, et al, Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci USA, 2010, 107(32), 14309-14314. [3]. Emami KH, et al. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected]. Proc Natl Acad Sci USA, 2004, 101(34), 12682-12687. [4]. Liu Y, et al. ICG-001 suppresses growth of gastric cancer cells and reduces chemoresistance of cancer stem cell-like population. J Exp Clin Cancer Res. 2017 Sep 11;36(1):125. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.