| Cas No.: | 1001753-24-7 |
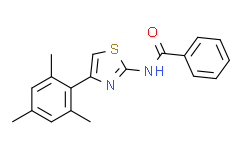
| Chemical Name: | N-[4-(2,4,6-trimethylphenyl)-1,3-thiazol-2-yl]benzamide |
| Synonyms: | N-(4-mesitylthiazol-2-yl)benzamide;INH6;N-[4-(2,4,6-trimethylphenyl)thiazol-2-yl]benzamide;N-[4-(2,4,6-trimethylphenyl)-2-thiazolyl]-benzamide;N-[4-(2,4,6-trimethylphenyl)-1,3-thiazol-2-yl]benzamide;C19H18N2OS;N-(4-(2,4,6-trimethylphenyl)thiazol-2-yl)benzamide;AMBZ0346;HMS3653H09;BCP11030;BQB75324;2569AH;s7494;AM85436 |
| SMILES: | S1C(N([H])C(C2C([H])=C([H])C([H])=C([H])C=2[H])=O)=NC(=C1[H])C1C(C([H])([H])[H])=C([H])C(C([H])([H])[H])=C([H])C=1C([H])([H])[H] |
| Formula: | C19H18N2Os |
| M.Wt: | 322.4240 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | INH6 is a potent Nek2/Hec1 inhibitor; inhibits the growth of HeLa cells with an IC50 of 2.4 μM. |
| In Vitro: | Hec1 is an oncogene overly expressed in many human cancers. Small molecule INH (Inhibitor of Nek2/Hec1) targeting the Hec1 and its regulator, Nek2, in the mitotic pathway is identified to inactivate Hec1/Nek2 function mediated by protein degradation that subsequently leads to chromosome mis-segregation and cell death. INH6 treated cells exhibit increased mitotic population with multipolar spindle configurations. An increased rate of chromosome misalignment is detected upon treatment with INH6 of HeLa cells expressing the chromosome marker protein H2B-GFP. INH6 treated cells shows progressive morphological changes characteristic of dying cells (e.g., membrane bubbling), which is further confirmed by cell cycle profiling with FACS analysis. Approximately 20% of INH6 treated cells are apoptotic 72 hrs after treatment[1]. |
| Cell Assay: | Standard XTT assays with a four-day drug treatment procedure were performed to measure the dose-dependent cytotoxicity of INH analogs in cultured cells. Triplicate sets were measured and compiled for final data presentation. Cells were plated on 96-well dishes one day before the drug treatment, followed by drug treatment (2.5 μM INH6) on day 2 and XTT assay on day 5 after drug addition. The absorption at 595 nm was measured with a plate reader and converted to cell survival percentages in comparison to mock treated groups[1]. |
| References: | [1]. Qiu XL, et al. Synthesis and biological evaluation of a series of novel inhibitor of Nek2/Hec1 analogues. J Med Chem. 2009 Mar 26;52(6):1757-67. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.