| Cas No.: | 1021497-97-1 |
| Chemical Name: | Unii-nch0QD81ZR |
| Synonyms: | KR-33494;KR 33494;4-(2-(4-bromophenylthio)acetamido)-1-phenethyl-1H-pyrazole-3-carboxylic acid;KR-33493;NCH0QD81ZR;4-[[2-[(4-Bromophenyl)thio]acetyl]amino]-1-(2-phenylethyl)-1H-pyrazole-3-carboxylic acid;BCP19823;4-((2-(4-Bromophenylsulfanyl)acetyl)amino)-1-phenethyl-1H-pyrazole-3-carboxylic acid;4-(2-((4-bromophenyl)thio)acetamido)-1-phenethyl-1H-pyrazole-3-carboxylic acid;4-[[2-(4-b;Unii-nch0QD81ZR |
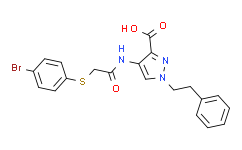
| SMILES: | BrC1C([H])=C([H])C(=C([H])C=1[H])SC([H])([H])C(N([H])C1C(C(=O)O[H])=NN(C=1[H])C([H])([H])C([H])([H])C1C([H])=C([H])C([H])=C([H])C=1[H])=O |
| Formula: | C20H18BrN3O3S |
| M.Wt: | 460.3442 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | KR-33493 is a potent inhibitor of Fas-mediated cell death (FAF1). |
| In Vivo: | Body weight changes of both sexes are not related to KR-33493 in all doses. In rats administrated KR-33493 for 4 weeks, no test article-related changes in any treated groups of either sex are found in hematology, serum biochemistry, and urinalysis. In dogs administrated KR-33493 for 2 weeks, red blood cell count (RBC) value in males is significantly higher at the 1000 mg/kg/day dose than that of the control group (i.e., 6.96±0.323 vs. 6.12±0.418). However, the change of RBC is recovered after the end of the administration period. The dose-normalized AUClast is not significantly different between the groups, suggesting that KR-33493 is governed by linear kinetics[1]. |
| Animal Administration: | A total of 93 male and 93 female specific pathogen-free rats (6 weeks of age), and 16 male and 16 female beagle dogs (8 months of age) are used in this study. In a toxicokinetic study, rat blood samples (approximately 0.6 mL) are collected into tubes containing heparin from the lateral tail vein at 0, 0.5, 1, 2, 4, 8, 12, and 24 h after dosing with KR-33493 at doses of 50, 150, and 500 mg/kg/day on Day 1 and Week 4. Dog blood samples (approximately 0.6 mL) are collected into tubes containing EDTA-2K from the cephalic vein at 0, 0.5, 1, 2, 4, 6, 8, and 24 h after dosing at KR-33493 doses of 50, 250, and 1000 mg/kg/day on Day 1 and Week 2. The plasma is separated by centrifugation (approximately 132,000 g, 3 min, 4°C) and stored at approximately -80°C until analysis. The KR-33493 concentration in plasma is quantified[1]. |
| References: | [1]. Jeong JW, et al. Subacute toxicity evaluation of KR-33493, FAF1 inhibitor for a new anti-parkinson's disease agent, after oral administration in rats and dogs. Regul Toxicol Pharmacol. 2016 Nov;81:387-396. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.