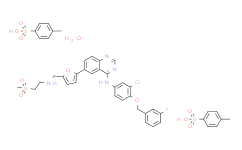
| Cas No.: | 388082-78-8 |
| Chemical Name: | Lapatinib |
| Synonyms: | Lapatinib Ditosylate;benzenesulfonic acid: N-[3-chloro-4-[(3-fluorophenyl)methoxy]phenyl]-6 -[5-[(2-methylsulfonylethylamino)methyl]-2-furyl]quinazolin-4-amine h ydrate;N-(3-Chloro-4-((3-fluorophenyl)methoxy)phenyl)-6-(5-(((2-(methylsulfonyl)ethyl)amino)methyl)-2-furanyl)-4-quinazolinamine bis(4-methylbenzenesulfonate);Tykerb N-{3-Chloro-4-[(3-fluorophenyl)-methoxy]-phenyl}-6-{5-{(2-methylsulfonylethy amino)-methyl]-2-furyl}-quinazolin-4-amine-benzenesulfonic acid;Lapatinib ditosylate hydrate;bis(N-(3-chloro-4-(3-fluorobenzyloxy)phenyl)-6-(5-((2-(methylsulfonyl)ethylamino)methyl)furan-...;Lapatinib; Lapatinib Ditosilate;Lapatinib ditosylate Monohydrate;4-Quinazolinamine,N-[3-chloro-4-[(3-fluorophenyl)methoxy]phenyl]-6-[5-[[[2-(methylsulfonyl)ethyl]amino]methyl]-2-furanyl]-,bis(4-methylbenzenesulfonate), monohydrate (9CI);N-(3-Chloro-4-((3-fluorophenyl)methoxy)phenyl)-6-(5-(((2-(methylsulfonyl)ethyl)amino)methyl)-2-furanyl)-4-quinazolinamine bis(4-methylbenzenesulfonate) monohydrate;Tykerb;GW572016;GW 572016;Lapatinib base;Lapatinib [INN];Tyverb;Lapatinib (INN);Lapatinib free base;GSK 572016;GSK572016;FMM;N-{3-CHLORO-4-[(3-FLUOROBENZYL)OXY]PHENYL}-6-[5-({[2-(METHYLSULFONYL)ETHYL]AMINO}METHYL)-2-FURYL]-4-QUINAZOLINAMINE;0VUA21238F;NSC745750;N-[3-chloro-4-[(3-fluorophenyl)methoxy]phenyl]-6-[5-[(2-methylsulfonylethylamino)methyl]furan-2-yl]quinazolin-4-amine;C29H26ClF |
| SMILES: | ClC1=C(C([H])=C([H])C(=C1[H])N([H])C1C2=C(C([H])=C([H])C(=C2[H])C2=C([H])C([H])=C(C([H])([H])N([H])C([H])([H])C([H])([H])S(C([H])([H])[H])(=O)=O)O2)N=C([H])N=1)OC([H])([H])C1C([H])=C([H])C([H])=C(C=1[H])F |
| Formula: | C43N4O11FS3ClH44 |
| M.Wt: | 943.4761 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Lapatinib ditosylate monohydrate (GW572016 ditosylate monohydrate) is a potent inhibitor of the ErbB-2 and EGFR tyrosine kinase domains with IC50 values against purified EGFR and ErbB-2 of 10.2 and 9.8 nM, respectively[1]. |
| In Vivo: | Lapatinib (GW2016; 30-100 mg/kg; oral administration; twice daily; for 21 days; CD-1 nude female mice) treatment inhibits tumor xenograft growth of the HN5 cells in a dose-responsive manner at 30 and 100 mg/kg, with complete inhibition of tumor growth at the higher dose[1]. Animal Model: CD-1 nude female mice (4-6 weeks old) with HN5 cells[1] Dosage: 30 mg/kg, 100 mg/kg Administration: Oral administration; twice daily; for 21 days Result: Inhibited tumor xenograft growth of the HN5 cells in a dose-responsive manner. |
| In Vitro: | Lapatinib (GW2016; 0.03-10 µM; 6 hours; BT474 and HN5 cells) treatment inhibits receptor autophosphorylation of EGFR and ErbB-2 in a dose-responsive manner. Phosphorylation of serine 473 of AKT was inhibited by GW2016 in a dose-dependent manner[1]. Lapatinib (GW2016; 72 hours; HN5, A-43, BT474, N87, and CaLu-3 cells) treatment has a selective inhibition of the proliferation of human tumor cell lines[1]. Lapatinib (GW2016; 1-10 µM; 72 hours; HN5 cells) treatment results in induces G1 arrest[1]. Western Blot Analysis[1] Cell Line: BT474 and HN5 cells Concentration: 0.03 µM, 0.1 µM, 0.3 µM, 1 µM, 3 µM, or 10 µM Incubation Time: 6 hours Result: Inhibited receptor autophosphorylation of EGFR and ErbB-2 in a dose-responsive manner. Phosphorylation of serine 473 of AKT was also inhibited in a dose-dependent manner. Cell Proliferation Assay[1] Cell Line: HN5, A-43, BT474, N87, and CaLu-3 cells Concentration: Incubation Time: 72 hours Result: Inhibited the growth of tumor cells overexpressing EGFR or ErbB-2. Cell Cycle Analysis[1] Cell Line: HN5 cells Concentration: 1 µM, or 10 µM Incubation Time: 72 hours Result: Resulted in induction of G1 arrest. |