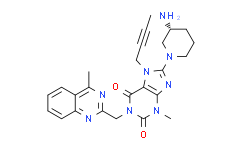
| Description: |
Linagliptin is a highly potent, selective DPP-4 inhibitor with IC50 of 1 nM. |
| In Vivo: |
In male Wistar rats, Beagle dogs, and Rhesus monkeys, xanthine linagliptin proves to be a highly efficacious, long-lasting, and potent DPP-4 inhibitor providing >70% inhibition for >7 h for all three species after oral administration of 1 mg/kg. Single oral administration of linagliptin to db/db mice 45 min prior to an oral glucose tolerance test reduced plasma glucose excursion in a dose-dependent manner from 0.1 mg/kg (15% inhibition) to 1 mg/kg (66% inhibition)[1]. Linagliptin (3 and 10 mg/kg) dose-dependently inhibits the DPP-4 enzyme in plasma within 30 min of administration. Linagliptin (1 mg/kg, p.o.) significantly reduces glucose excursion by approximately 50%[2]. Oral administration of the DPP-4 inhibitor linagliptin (3 mg/kg, p.o.) strongly reduces DPP-4 activity, stabilizes active GLP-1 in chronic wounds, and improves healing in ob/ob mice. At day 10 postwounding, linagliptin-treated ob/ob mice show largely epithelialized wounds characterized by the absence of neutrophils[3]. |
| In Vitro: |
Linagliptin inhibits DPP-4 activity in vitro in several independent experiments with IC50 values of 0.4, 0.5, 0.9, and 1.1 nM (mean IC50, approximately 1 nM). Linagliptin inhibits FAP with an IC50 of 89 nM (approximately 90-fold selectivity versus DPP-4)[2]. |
| Kinase Assay: |
EDTA plasma (20 μL) is diluted with 30 μL of DPP-4 assay buffer (100 mM Tris and 100 mM NaCl, adjusted to pH 7.8 with HCl) and mixed with 50 μL of H-Ala-Pro-7-amido-4-trifluoromethylcoumarin. The 200 mM stock solution in dimethylformamide is diluted 1:1000 with water to yield a final concentration of 100 μM. The plate is incubated at room temperature for 10 min, and fluorescence in the wells is determined by using a Victor 1420 Multilabel Counter at an excitation wavelength of 405 nm and an emission wavelength of 535 nm. For the detection of DPP-4 activity in wound lysates, 100 μg of protein from the respective wound lysates are used instead of 20 μL of plasma. Active GLP-1 is also detected from 100 μg of respective wound tissue samples and analyzed by using the Mouse/Rat Total Active GLP-1 Assay Kit. |
| Cell Assay: |
A total of 4.0×107 keratinocytes per well are seeded into 24-well plates. After reaching 50% confluence, cells are starved for 24 h with DMEM. Proliferation of cells is assessed by using 1 μCi/mL of [3H]methyl-thymidine in DMEM in the presence of 10% fetal bovine serum and increasing concentrations of linagliptin (3, 30, 300, or 600 nM) for 24 h. Cells are then washed twice with phosphate-buffered saline and incubated in 5% trichloroacetic acid at 4°C for 30 min, and the DNA is solubilized in 0.5mol/LNaOH for 30 min at 37°C. Finally, [3H]thymidine incorporation is determined. |
| Animal Administration: |
Each experimental group (vehicle or linagliptin treatment) consists of 10 individual ob/ob mice (n=10). Animals are treated orally once a day (8:00 AM) by gastrogavage using vehicle (1% methylcellulose) or linagliptin (3 mg/kg body weight in 1% methylcellulose) beginning 2 days (day−2) before wounding. After wounding, animals are subsequently treated once a day throughout the 10-day healing period. |
| References: |
[1]. Eckhardt M, et al. 8-(3-(R)-aminopiperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3,7-dihydropurine-2,6-dione (BI 1356), a highly potent, selective, long-acting, and orally bioavailable DPP-4 inhibitor for the treatment of type 2 d
[2]. Thomas L, et al. (R)-8-(3-amino-piperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3,7-dihydro-purine-2,6-dione (BI 1356), a novel xanthine-based dipeptidyl peptidase 4 inhibitor, has a superior potency and longer duration of action
[3]. Schurmann C, et al. The dipeptidyl peptidase-4 inhibitor linagliptin attenuates inflammation and accelerates epithelialization in wounds of diabetic ob/ob mice. J Pharmacol Exp Ther. 2012 Jul;342(1):71-80.
[4]. Huan Y, et al. The dual DPP4 inhibitor and GPR119 agonist HBK001 regulates glycemic control and beta cell function ex and in vivo. Sci Rep. 2017 Jun 28;7(1):4351. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.