| Cas No.: | 115550-35-1 |
| Chemical Name: | Marbofloxacin |
| Synonyms: | Marbofloxacin;9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-piperazino)-7-oxo-7h-pyrido[1,2,3-ij][1,2,4]benzoxadiazine-6-carboxylic acid;9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[3,2,1-ij][4,1,2]benzoxadiazine-6-carboxylic acid;Marbocyl;Marbofloxacin (INN);Marbofloxacino [INN-Spanish];UNII-8X09WU898T;Zeniquin;Zeniquin®;9-Fluoro-3,7-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-2H-[1,3,4]oxadiazino[6,5,4-ij]quinoline-6-carboxylic Acid;ZENIQUIN(R);Marbofloxacine;Marbofloxacin, Vetranal;Marbofloxacin Solution, 100ppm;Marbofloxacin Solution, 1000ppm;o-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-;Marbofloxacine [INN-French];Marbofloxacinum [INN-Latin];BPFYOAJNDMUVBL-UHFFFAOYSA-N;8X09WU898T;AK327608;DSSTox_CID_26600;DSSTox_RID_81755;DSSTox_GSID_46600;7H-Pyrido[3,2,1-ij][4,1,2]benzoxadiazine-6-carboxylic acid, 9-fluoro-2,3-dihydro-3-methy |
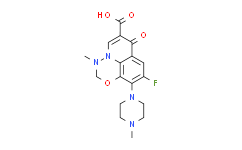
| SMILES: | CN1CCN(CC1)C2=C3C4=C(C=C2F)C(=O)C(C(O)=O)=CN4N(C)CO3 |
| Formula: | C17H19FN4O4 |
| M.Wt: | 362.3556 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Marbofloxacin is a potent antibiotic of which depends upon its inhibition of DNA-gyrase. Marbofloxacin is a synthetic, broad spectrum bactericidal agent.Marbofloxacin is a third-generation fluoroquinolone for veterinary use, the antimicrobial of which depends upon its inhibition of DNA-gyrase and topoisomerase IV. With a broad spectrum bactericidal activity and good efficacy, marbofloxacin is indicated for dermatological, respiratory and urinary tract infections due to both Gram-positive and Gram-negative bacteria and Mycoplasma [1].Administration of Marbofloxacin at 6 mg/kg once daily for 7 days in a Staphylococcus aureus infection in tissue cages in ponies is not effective for the elimination of S. aureus infections from secluded sites [2]. The pharmacokinetic properties of marbofloxacin were investigated in 6 horses after i.v., subcutaneous and oral administration of a single dose of 2 mg/kg bwt and the minimal inhibitory concentrations (MIC) assessed for bacteria isolated from equine infectious pathologies. The clearance of marbofloxacin was mean +/- s.d. 0.25 +/- 0.05 l/kg/h and the terminal half-life 756 +/- 1.99 h. The marbofloxacin absolute bioavailabilities after subcutaneous and oral administration were 98 +/- 11% and 62 +/- 8%, respectively. Considering the breakpoint values of efficacy indices for fluoroquinolones, a marbofloxacin dosage regimen of 2 mg/kg bwt/24 h by i.v., subcutaneous or oral routes was more appropriate for enterobacteriaceae than for S. aureus [3]. Toxicity: cramps; vomiting; anorexia; soft stools; diarrhoea |
| Target: | DNA-gyrase |
| References: | [1]. Shen J, et al. Marbofloxacin. Acta Crystallogr Sect E Struct Rep Online. 2012 Apr 1;68(Pt 4):o998-9. [2]. Voermans M, et al. Clinical efficacy of intravenous administration of marbofloxacin in a Staphylococcus aureus infection in tissue cages in ponies. J Vet Pharmacol Ther. 2006 Dec;29(6):555-60. [3]. Bousquet-Melou A, et al. Pharmacokinetics of marbofloxacin in horses. Equine Vet J. 2002 Jul;34(4):366-72. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.