| Cas No.: | 198821-22-6 |
| Chemical Name: | Merimepodib |
| Synonyms: | [(3S)-oxolan-3-yl] N-[[3-[[3-methoxy-4-(1,3-oxazol-5-yl)phenyl]carbamoylamino]phenyl]methyl]carbamate;MERIMEPODIB;(3S)-tetrahydrofuran-3-yl [3-({[3-methoxy-4-(1,3-oxazol-5-yl)phenyl]carbamoyl}amino)benzyl]carbamate;MMP;VI21497;VI-21497;VX497;VX-497;[[3-[[[[3-Methoxy-4-(5-oxazolyl)phenyl]amino]carbonyl]amino]phenyl]methyl]carbamic acid (3S)-tetrahydro-3-furanyl ester;MeriMepodib, VI-21497, VX-497;Carbamic acid, N-[[3-[[[[3-methoxy-4-(5-oxazolyl)phenyl]amino]carbonyl]amino]phenyl]methyl]-, (3S)-tetrahydro-3-furanyl ester;VI 21497;VX 497;2ZL2BA06FU;C23H24N4O6;(S)-tetrahydrofuran-3-yl 3-(3-(3-methoxy-4-(oxazol-5-yl)phenyl)ureido)benzylcarbamate;MMPD;Merimepodib [USAN:INN];Merimebodib;carbamic acid, [[3-[[[[3-methoxy-4-(5-oxazolyl)phenyl]amino]carbonyl]amino]phenyl]methyl]-, (3S)-tetrahydro-3-furanyl ester;Carbamic acid, |
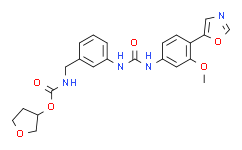
| SMILES: | O1C([H])([H])C([H])([H])[C@@]([H])(C1([H])[H])OC(N([H])C([H])([H])C1C([H])=C([H])C([H])=C(C=1[H])N([H])C(N([H])C1C([H])=C([H])C(C2=C([H])N=C([H])O2)=C(C=1[H])OC([H])([H])[H])=O)=O |
| Formula: | C23N4O6H24 |
| M.Wt: | 452.4599 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Merimepodib is a novel noncompetitive inhibitor of IMPDH (Inosine monophosphate dehydrogenase).. |
| In Vivo: | Oral administration of VX-497 inhibits the primary IgM antibody response in a dose-dependent manner, with an ED50 value of appr 30-35 mg/kg in mice. Single daily dosing of VX-497 is observed to be as effective as twice-daily dosing in this model of immune activation[1]. GVHD developed in the vehicle-treated allografted F1 mice and treatment with VX-497 improved all manifestations of the disease significantly. The 2.9-fold increase in spleen weight in allografted animals is reduced to a 1.6-fold increase in the VX-497-treated mice. Serum IFN-gamma levels are increased 54-fold in the vehicle group while there is a 7.4-fold increase in VX-497-treated animals[3]. |
| In Vitro: | VX-497 has antiproliferative effect on lymphoid and keratinocyte cells. The antiproliferative effect of VX-497 in cells is reversed within 48 h of its removal[1]. VX-497 has intermediate antiviral activity against a second group of viruses, which includes HSV-1, parainfluenza-3 virus, BVDV, VEEV, and dengue virus, with IC50s ranging from 6 to 19 μM. VX-497 is 100-fold more potent, with an IC50 of 380 nM and a corresponding CC50 of 5.2 μM, for a therapeutic index of 14. The antiviral activity of VX-497 in HepG2.2.2.15 cells is reversed threefold by the addition of guanosine[2]. |
| Cell Assay: | The murine fibroblast L929 cell line is cultured in Eagle minimal essential medium supplemented with 10% fetal bovine serum, nonessential amino acids, 50 U of penicillin per mL, 50 μg of streptomycin per mL, and 2 mM l-glutamine. EMCV is infected at 500 PFU/107 L929 cells. Cells are left untreated or are treated with different concentrations of murine IFN-α alone, VX-497 alone, or combinations thereof. |
| References: | [1]. Jain J, et al. VX-497: a novel, selective IMPDH inhibitor and immunosuppressive agent. J Pharm Sci. 2001 May;90(5):625-37. [2]. Markland W, et al. Broad-spectrum antiviral activity of the IMP dehydrogenase inhibitor VX-497: a comparison with ribavirin and demonstration of antiviral additivity with alpha interferon. Antimicrob Agents Chemother. 2000 Apr;44(4):859-66. [3]. Decker CJ, et al. The novel IMPDH inhibitor VX-497 prolongs skin graft survival and improves graft versus host disease in mice. Drugs Exp Clin Res. 2001;27(3):89-95. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.