| Cas No.: | 878141-96-9 |
| Synonyms: | E-52862; E52862; E 52862 |
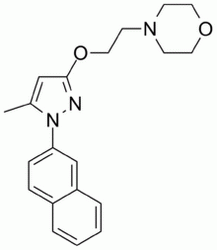
| SMILES: | CC1=CC(=NN1C2=CC3=CC=CC=C3C=C2)OCCN4CCOCC4 |
| Formula: | C20H23N3O2 |
| M.Wt: | 337.42 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | S1RA(E-52862) is a potent and selective sigma-1 receptor(σ1R, Ki=17 nM) antagonist, showed good selectivity against σ2R (Ki > 1000 nM). |
| Target: | IC50 value: 17 nM (Ki) [1] Target: σ1R |
| In Vivo: | Control (non-operated) and nerve-injured mice received a single or repeated (twice daily for 12 days) i.p. administration of S1RA at 25 mg·kg?1, the same dose used for the assessment of behavioural hypersensitivity in the chronic treatment study. Acute treatment was given on day 12 post-surgery and repeated treatment with S1RA started the day of surgery, as in the behavioural studies [2]. Intrathecal pre-treatment with idazoxan prevented the systemic S1RA antinociceptive effect, suggesting that the S1RA antinociception depends on the activation of spinal α2 -adrenoceptors which, in turn, could induce an inhibition of formalin-evoked glutamate release. When administered locally, intrathecal S1RA inhibited only the flinching behavior, whereas intracerebroventricularly or intraplantarly injected also attenuated the lifting/licking behavior [3]. |
| In Vitro: | S1RA behaved as a highly selective σ1 receptor antagonist. It showed high affinity for human (Ki= 17 nM) and guinea pig (Ki= 23.5 nM) σ1 receptors but no significant affinity for the σ2 receptors (Ki > 1000 nM for guinea pig and rat σ2 receptors). Moderate affinity (Ki= 328 nM) and antagonistic activity, with very low potency (IC50= 4700 nM) was found at the human 5-HT2B receptor. S1RA showed no significant affinity (Ki > 1 μM or % inhibition at 1 μM < 50%) for other additional 170 targets (receptors, transporters, ion channels and enzymes) [2]. |
| References: | [1]. Díaz JL, et al. Synthesis and biological evaluation of the 1-arylpyrazole class of σ(1) receptor antagonists: identification of 4-{2-[5-methyl-1-(naphthalen-2-yl)-1H-pyrazol-3-yloxy]ethyl}morpholine (S1RA, E-52862). J Med Chem. 2012 Oct 11;55(19):8211-24. [2]. Romero L, et al. Pharmacological properties of S1RA, a new sigma-1 receptor antagonist that inhibits neuropathic pain and activity-induced spinal sensitization. Br J Pharmacol. 2012 Aug;166(8):2289-306. [3]. Vidal-Torres A, et al. Effects of the selective sigma-1 receptor antagonist S1RA on formalin-induced pain behavior and neurotransmitter release in the spinal cord in rats. J Neurochem. 2014 Jan 3. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.