| Cas No.: | 14003-96-4 |
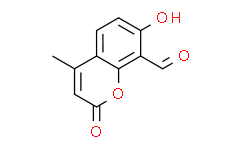
| Chemical Name: | 7-Hydroxy-4-methyl-2-oxo-2H-chromene-8-carbaldehyde |
| Synonyms: | 7-Hydroxy-4-methyl-2-oxo-2H-chromene-8-carbaldehyde;4Μ8C;7-hydroxy-4-methyl-2-oxochromene-8-carbaldehyde;8-Formyl-4-methylumbelliferone;IRE1 Inhibitor III;4μ7-Hydroxy-4-methyl-2-oxo-2H-1-benzopyran-8-carboxaldehyde;4-Methyl-7-hydroxy-8-formylcoumarin;7-Hydroxy-8-formyl-4-methylcoumarin;8-Formyl-7-hydroxy-4-methyl-2H-1-benzopyran-2-one;4M8C;4u8C;4μIRE1 Inhibitor III, 4μ8C;8-Formyl-7-hydroxy-4-methylcoumarin;4;I8C;4mu8C;2H-1-Benzopyran-8-carboxaldehyde, 7-hydroxy-4-methyl-2-oxo-;4micro8C;HMS3743K09;HMS3653K06;BCP21011;STK504078;s7272;4150AD;BDBM5001379 |
| SMILES: | O1C(C([H])=C(C([H])([H])[H])C2C([H])=C([H])C(=C(C([H])=O)C1=2)O[H])=O |
| Formula: | C11H8O4 |
| M.Wt: | 204.1788 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | 4μ8C (IRE1 Inhibitor III) is a small-molecule inhibitor of IRE1α. |
| In Vivo: | 4μ8C reverses the ER stress-dependent loss of several known RIDD targets, with an EC50 of approximately 4 μM, approximating that of inhibition of XBP1 target gene activation[1]. |
| In Vitro: | When applies to the media of ER stressed cultured cells, 4μ8C inhibits Xbp1 splicing in a concentration-dependent manner. 4μ8C dissociates slowly from IRE1, but ishout of inhibitor leads to rapid recovery of Xbp1 splicing in cells[1].The IRE1 endoribonuclease inhibitor 4μ8c prevents the splicing of the XBP1 mRNA in response to ER stress caused by mutant proinsulin production[2]. The inositol-requiring enzyme 1α (IRE1α) is a serine-threonine kinase that plays crucial roles in activating the unfolded protein response. 4μ8C treatment dramatically inhibits IL-4 production by CD4+ T cells under Th0 conditions because both the IL-4 levels in the culture supernatant and the percentage of IL-4 positive cells are reduced by 4μ8C treatment. In addition, both IL-5 and IL-13 production are significantly reduced upon treatment with 4μ8C[3]. |