| Cas No.: | 5928-25-6 |
| Chemical Name: | Decursin |
| Synonyms: | DECURSIN;DECURSINOL ANGELATE;(7S)-7,8-Dihydro-8,8-dimethyl-7-[(3-methyl-2-butenoyl)oxy]-2H,6H-benzo[1,2-b:5,4-b']dipyran-2-one;3-Methyl-2-butenoic acid (7S)-7,8-dihydro-8,8-dimethyl-2-oxo-2H,6H-benzo[1,2-b:5,4-b']dipyran-7-yl ester;3-Methyl-2-butenoic acid (7S)-7,8-dihydro-8,8-dimethyl-2-oxo-2H,6H-pyrano[3,2-g]-1-benzopyran-7-yl ester;2-Butenoic acid,3-methyl-,(7S)-7,8-dihydro-8,8-dimethyl-2-oxo-2H,6H-pyrano[3,2-g]-1-benzopyran...;8,8-Dimethyl-2-oxo-7,8-dihydro-2H,6H-pyrano[3,2-g]chromen-7-yl 3-methyl-2-butenoate-, (S)-;(+)-Decursin;(7S)-7,8-Dihydro-8,8-dimethyl-2-oxo-2H,6H-benzo[1,2-b:5,4-b′]dipyran-7-yl 3-methyl-2-butenoate;(S)-(+)-Decursin;E95RTO3YQR;[(3S)-2,2-dimethyl-8-oxo-3,4-dihydropyrano[3,2-g]chromen-3-yl] 3-methylbut-2-enoate;BDBM50361396;9318AF;Decursin (Synonyms: (+)-Decursin);API0002231;(S)-7,8-Dihydro-8,8-dimethyl-2-oxo-2H,6H-benzo(1,2-b:5,4-b')dipyran-7-yl 3-methyl-2-butenoate;C09258;(S)-2,2-dimethyl-8-oxo-2,3,4,8-tetrahy;Decursin ((+)-Decursin);BCP10293;s9264;Q27106347;(S)-2,2-dimethyl-8-oxo- |
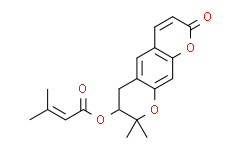
| SMILES: | O1C2C([H])=C3C(C([H])=C([H])C(=O)O3)=C([H])C=2C([H])([H])[C@@]([H])(C1(C([H])([H])[H])C([H])([H])[H])OC(/C(/[H])=C(\C([H])([H])[H])/C([H])([H])[H])=O |
| Formula: | C19H20O5 |
| M.Wt: | 328.3591 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Decursin is an anticancer agent, with potential anti-inflammatory activity. |
| In Vitro: | Decursin (25-100 μM) shows a strong dose- and time-dependent inhibition of cell growth, accounting for 22% to 51%, 21% to 68%, 9% to 72%, and 42% to 90% growth inhibition after 24, 48, 72, and 96 hours of treatment, respectively. Furthermore, it is observed that both Decursin and Decursinol have cytotoxic effect on DU145 cells, in which similar treatment (25-100 μM for 24, 48, 72, and 96 hours) with Decursin cause 15% to 45% cell death versus 11% to 12% in controls[1]. Decursin blocks tumor progression by suppression of angiopoietin-2, angiopoietin receptor Tie-2, and endothelial nitric oxide synthase (eNOS) in endothelial progenitor cells. Decursin has the ability to inhibit vascular endothelial growth factor (VEGF)-induced proliferation, metastasis, and tube formation in human umbilical vein endothelial cells (HUVECs). Decursin inhibits pro-inflammatory molecules, such as chemokines, cytokines, and enzymes such as Cyclooxygenase-2 (COX-2) and MMPs[2]. |
| Cell Assay: | To assess the biological activity of these compounds in terms of cell growth and death, DU145 cells are treated with 25, 50, and 100 μM doses of Decursin and Decursinol for 24, 48, 72, and 96 hours[1]. |
| References: | [1]. Yim D, et al. A novel anticancer agent, decursin, induces G1 arrest and apoptosis in human prostate carcinoma cells. Cancer Res. 2005 Feb 1;65(3):1035-44. [2]. Shehzad A, et al. Decursin and decursinol angelate: molecular mechanism and therapeutic potential in inflammatory diseases. Inflamm Res. 2018 Mar;67(3):209-218. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.