| Description: |
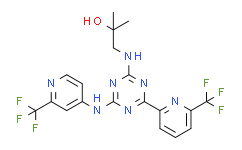
Enasidenib is an oral, potent, reversible, selective inhibitor of the IDH2 mutant enzymes, with IC50s of 100 and 400 nM against IDH2R140Q and IDH2R172K, respectively. |
| In Vivo: |
Treatment with Enasidenib (AG-221) significantly improves survival in an IDH2-mutant acute myeloid leukemia (AML) primary xenograft mouse model[1]. Enasidenib (AG-221), a mutant IDH2 inhibitor, remodels the epigenetic state of IDH2-mutant cells and induces alterations in self-renewal/differentiation in IDH2-mutant AML model in vivo. Enasidenib treatment (10mg/kg or 100mg/kg bid) leads to a reduction in 2-HG in vivo (96.7% below pre-treatment levels). Moreover, Enasidenib treatment restores megakaryocyte-erythroid progenitor (MEP) differentiation that is suppressed by mutant IDH2 expression (mean MEP% mean, 39% Veh vs 50% AG-221). Enasidenib therapy reverses the effects of mutant IDH2; a significant reduction is observed in DNA methylation, including 180 genes that have 20 or more hypomethylated differentially methylated cytosines (DMCs) following treatment. Enasidenib (100mg/kg bid) treatment of mice engrafted with Mx1-Cre IDH2R140QFlt3ITD AML cells markedly reduces 2-hydroxyglutarate (2-HG) levels consistent with on target inhibition. Enasidenib inhibits mutant IDH2-mediated 2-HG production[2]. |
| In Vitro: |
Enasidenib (AG-221) reverses the effects of mutant IDH2 on DNA methylation in mutant stem/progenitor cells. Enasidenib induces differentiation and impairs self-renewal of IDH2-mutant leukemia cells, effects that are further enhanced by simultaneous inhibition of Flt3ITD. Enasidenib (AG-221) therapy induces differentiation of leukemic cells, with an increase in the CD11b+ population and a decrease in the c-Kit+ population in the peripheral blood at 2wks[2]. |