| Cas No.: | 796-42-9 |
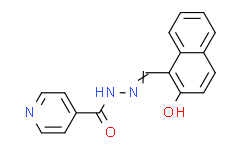
| Chemical Name: | N'-[(Z)-(2-Oxonaphthalen-1-ylidene)methyl]pyridine-4-carbohydrazide |
| Synonyms: | 2-hydroxy-1-naphthylaldehyde Isonicotinoyl Hydrazone;(E)-N'-((2-hydroxynaphthalen-1-yl)methylene)isonicotinohydrazide;NSC525357;1-isonicotinyl-2-(2-hydroxy-1-naphthylidene) hydrazine;isonicotinic acid-[(2-hydroxy-[1]naphthylmethylene)-hydrazide];1-Isonicotinoyl-5-(4-nitro-phenyl)-carbohydrazid;2-hydroxy-1-naphthyl aldehyde isonicotinoyl hydrazone;AC1MPQ3B;2-hydroxy-1-napthaldehyde isonicotinoylhydrazone;Isonicotinsaeure-[(2-hydroxy-[1]naphthylmethylen)-hydrazid];(E)-N'-((2-hydroxynaphthalen-1-yl)methylene)isonicotinohydrazide; NSC525357; 1-isonicotinyl-2-(2-hydroxy-1-naphthylidene) hydrazine; isonicotinic acid-[(2-hydroxy-[1]naphthylmethylene)-hydrazide]; 1-Isonicotinoyl-5-(4-nitro-phenyl)-carbohydrazid; 2-hydroxy-1-naphthyl aldehyde isonicotinoyl hydrazone; AC1MPQ3B; 2-hydroxy-1-napthaldehyde isonicotinoylhydrazone; Isonicotinsaeure-[(2-hydroxy-[1]naphthylmethylen)-hydrazid]; 1-Isonicotinoyl-5;2-Hydroxy-1-naphthylaldehyde isonicotinoylhydrazone;311 Iron Chelator;AS-8351;NSC-51355;AS8351;N'-[(2-hydroxy-1-naphthyl)methylene]isonicotinohydrazide;N'-[(1E)-(2-hydroxynaphthalen-1-yl)methylidene]pyridine-4-carbohydrazide;NSC51355;N'-[(2-hydroxynaphthalen-1-yl)methylidene]pyridine-4-carbohydrazide;2- HYDROXY-1-NAPHTHALDEHYDE-ISONICOTINOYLHYDRAZONE;N'-[(Z)-(2-oxonaphthalen-1-ylidene)methyl]pyridine-4-carbohydrazide;STK037070;NE57591;AS 8351 |
| SMILES: | O([H])C1C([H])=C([H])C2=C([H])C([H])=C([H])C([H])=C2C=1/C(/[H])=N/N([H])C(C1C([H])=C([H])N=C([H])C=1[H])=O |
| Formula: | C17H13N3O2 |
| M.Wt: | 291.3040 |
| Purity: | >98% |
| Sotrage: | 4°C for 1 year, -20°C for more than 2 years |
| Description: | AS8351 is a KDM5B inhibitor, which can induce and sustain active chromatin marks to facilitate the induction of cardiomyocyte-like cells. |
| In Vitro: | AS8351 affects epigenetic modifications by competing with α-ketoglutarate (α-KG) for chelating iron [Fe(II)] in certain epigenetic enzymes, such as the JmjC domain-containing histone demethylases (JmjC-KDMs) that require α-KG and iron as co-factors[2]. |
| References: | [1]. Liu K, et al. Chemical Modulation of Cell Fate in Stem Cell Therapeutics and Regenerative Medicine. Cell Chem Biol. 2016 Aug 18;23(8):893-916. [2]. Cao N, et al. Conversion of human fibroblasts into functional cardiomyocytes by small molecules. Science. 2016 Jun 3;352(6290):1216-20. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.