| Cas No.: | 1420477-60-6 |
| Chemical Name: | Acalabrutinib (ACP-196) |
| Synonyms: | Acalabrutinib;Calquence;I42748ELQW;Benzamide, 4-(8-amino-3-((2S)-1-(1-oxo-2-butyn-1-yl)-2-pyrrolidinyl)imidazo(1,5-a)pyrazin-1-yl)-N-2-pyridinyl-;Acalabrutinib [INN];Acalabrutinib [USAN:INN];Calquence (TN);Benzamide, 4-[8-amino-3-[(2S)-1-(1-oxo-2-butyn-1-yl)-2-pyrrolidinyl]imidazo[1,5-a]pyrazin-1-yl]-N-2-pyridinyl-;Acalabrutinib(ACP196);GTPL8912;Acalabrutinib (JAN/USAN/INN);EX;Acalabrutinib(ACP-196) |
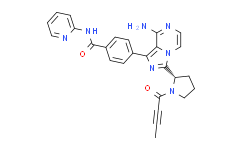
| SMILES: | O=C(C#CC([H])([H])[H])N1C([H])([H])C([H])([H])C([H])([H])[C@@]1([H])C1=NC(C2C([H])=C([H])C(C(N([H])C3=C([H])C([H])=C([H])C([H])=N3)=O)=C([H])C=2[H])=C2C(N([H])[H])=NC([H])=C([H])N12 |
| Formula: | C26H23N7O2 |
| M.Wt: | 465.52 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Acalabrutinib is a novel, potent, and highly selective BTK inhibitor, with an IC50 of 3 nM and EC50 of 8 nM in in vitro assay. |
| In Vivo: | In the human CLL NSG xenograft model, acalabrutinib demonstrates on-target effects including decreased phosphorylation of PLCγ2, ERK and significant inhibition of CLL cell proliferation. Acalabrutinib significantly decreases tumor burden in the spleen of the mice. In the TCL1 adoptive transfer model, acalabrutinib treatment decreases phosphorylation of BTK, PLCγ2 and S6. Most notably, acalabrutinib results in a significant increase in survival compared to mice receiving vehicle[1]. Acalabrutinib (100 mg twice per day) assessed for thrombus formation at injured arterioles of the mice, exhibits more selective for inhibiting BTK and has virtually no inhibition of platelet activity[2]. |
| In Vitro: | IC50: 3 nM (BTK in CD69 B cell)[2] |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.