| Cas No.: | 73069-13-3 |
| Chemical Name: | Atractylenolide I |
| Synonyms: | Atractylenolide-1;(4aS,8aS)-4a,5,6,7,8,8a-Hexahydro-3,8a-dimethyl-5-methylenenaphtho[2,3-b]furan-2(4H)-one;3,8aβ-Dimethyl-5-methylene-2,4,4aα,5,6,7,8,8a-octahydronaphtho[2,3-b]furan-2-one;Eudesma-4(15),7(11),8-trien-12-olide;Atractylenolide I;Atractylenolide-I;Naphtho[2,3-b]furan-2(4H)-one,4a,5,6,7,8,8a-hexahydro-3,8a-dimethyl-5-methylene-, (4aS,8aS)-;The extracted oil;Atractylenolide 1;AtractylenolideI;(4aS,8aS)-3,8a-dimethyl-5-methylidene-4a,6,7,8-tetrahydro-4H-benzo[f][1]benzofuran-2-one;BDBM50241939;s8291;Atractylenolide I, >=98% (HPLC);N2541;X1093;C17885;069A133;Naphtho[2,3-b]furan-2(4H)-one,4a,5,6,7,8,8a-hexahydro-3,8a-dimethyl-5-m |
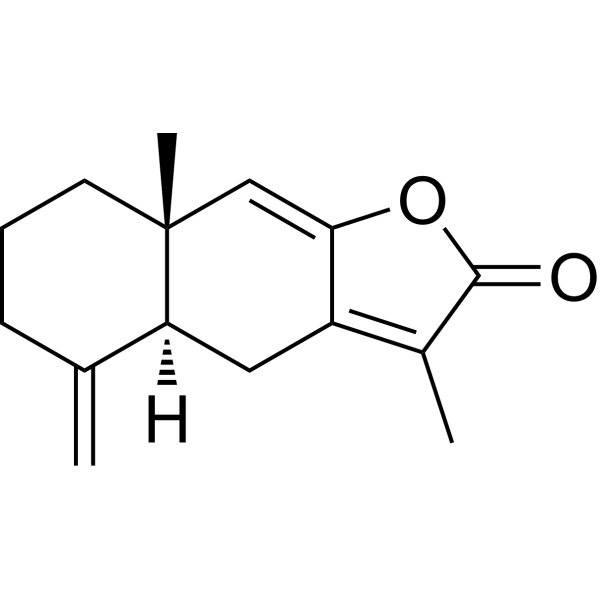
| SMILES: | O=C1C(C)=C(C[C@@]23[H])C(O1)=C[C@@]3(C)CCCC2=C |
| Formula: | C15H18O2 |
| M.Wt: | 230.3022 |
| Purity: | >98%, Standard References Grade |
| Sotrage: | 4°C for 1 year, -20°C for more than 2 years |
| Description: | Atractylenolide I is a sesquiterpene derived from the rhizome of Atractylodes macrocephala, possesses diverse bioactivities, such as neuroprotective, anti-allergic, anti-inflammatory and anticancer properties. Atractylenolide I reduces protein levels of phosphorylated JAK2 and STAT3 in A375 cells, and acts as a TLR4-antagonizing agent. |
| In Vivo: | Atractylenolide I (5, 10 or 20 mg/kg, p.o.) restores the decreased body weight in mice subjected to chronic unpredictable mild stress (CUMS). Atractylenolide I alleviates CUMS-induced depressive-like behavior, attenuates CUMS-induced imbalances in hippocampal neurotransmitter levels and reduces CUMS-induced increases in hippocampal pro-inflammatory cytokine levels and in the NLRP3 inflammasome in the hippocampi of mice[2]. |
| In Vitro: | Atractylenolide I (40, 60, 80, 100, 120, 150 μM) dose- and time-dependently reduces the cell viability in human A375 melanoma cells after treatment for 24, 48 and 72 hours. Atractylenolide I (50 and 100 μM) induces apoptosis of A375 cells in a dose-dependent manner at 48 h of treatment. Atractylenolide I (100 μM) significantly reduces protein levels of phosphorylated JAK2 and STAT3 in A375 cells, without effect on total JAK2 and STAT3. Furthermore, Atractylenolide I inhibits the mRNA expression of STAT3-targeted genes, including Bcl-xL, MMP-2 and MMP-9[1]. Atractylenolide I (up to 100 μM) shows no toxicity in normal cells. Atractylenolide I (25, 50 μM) decreases the Ox-LDL induced TNF-α, IL-6 and NO production in VSMCs. Atractylenolide I (12.5, 25 or 50 μM) significantly reduces the level of MCP-1 and inhibits Ox-LDL-induced VSMCs proliferation and migration. Atractylenolide I (25, 50 μM) inhibits positive staining of foam cells, and also significantly decreases lipid accumulation. Atractylenolide I (50 μM) suppresses p38MAPK and NF-κB p65 expression in VSMCs stimulated by Ox-LDL[3]. Atractylenolide I (1, 10, 100 μM) downregulates paclitaxel-induced expression of VEGF and survivin via MyD88-dependent TLR4 signaling in EOC cells[4]. |
| Cell Assay: | Briefly, serum starved VSMCs are pre-treated with indicated concentration of Atractylenolide I for 1 h followed by stimulation with Ox-LDL for 24 h. The purple formazan crystals formed after addition of MTT are solubilized in DMSO and absorbance is measured at 540 nm. The viability or proliferation rate is calculated as percentage of control (untreated VSMCs)[3]. |
| Animal Administration: | Mice[2] After adaption for one week, 48 male ICR mice are randomly divided into six groups (eight mice per group): Control group (unstressed + saline vehicle), model group (CUMS + saline vehicle), three Atractylenolide I treatment groups (CUMS + Atractylenolide I) and a fluoxetine group (CUMS + FLU). From the 4th week, Atractylenolide I (5, 10 or 20 mg/kg) or fluoxetine (20 mg/kg) is daily administered by oral gavage for 3 weeks. After the last administration of Atractylenolide I or fluoxetine, behavioral tests are performed[2]. |
| References: | [1]. Atractylenolide I, et al. The JAK2/STAT3 pathway is involved in the anti-melanoma effects of atractylenolide I. Exp Dermatol. 2018 Feb;27(2):201-204. [2]. Gao H, et al. Anti-depressant-like effect of atractylenolide I in a mouse model of depression induced by chronic unpredictable mild stress. Exp Ther Med. 2018 Feb;15(2):1574-1579. [3]. Li W, et al. Atractylenolide I restores HO-1 expression and inhibits Ox-LDL-induced VSMCs proliferation, migration and inflammatory responses in vitro. Exp Cell Res. 2017 Apr 1;353(1):26-34. [4]. Huang JM, et al. Atractylenolide-I sensitizes human ovarian cancer cells to paclitaxel by blocking activation of TLR4/MyD88-dependent pathway. Sci Rep. 2014 Jan 23;4:3840. |