| Cas No.: | 570403-17-7 |
| Chemical Name: | Avatrombopag hydrochloride |
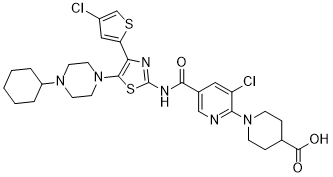
| SMILES: | O=C(C1CCN(C2=NC=C(C(NC3=NC(C4=CC(Cl)=CS4)=C(N5CCN(C6CCCCC6)CC5)S3)=O)C=C2Cl)CC1)O.Cl |
| Formula: | C29H35Cl3N6O3S2 |
| M.Wt: | 686.12 |
| Purity: | >98% |
| Sotrage: | Powder-20°C3 years4°C2 yearsIn solvent-80°C6 months-20°C1 month |
| Description: | Avatrombopag (AKR-501) hydrochloride is an orally active, nonpeptide thrombopoietin (TPO) receptor agonist (EC50=3.3 nM). Avatrombopag hydrochloride mimics the biological activities of TPO. Avatrombopag hydrochloride increases platelet production by activating the intracellular signaling system, and promotes production of platelets and megakaryocytes from hemopoietic precursor cells. Avatrombopag hydrochloride is a substrate of cytochrome P450 (CYP) 2C9 and CYP3A[1][2][3]. |
| In Vivo: | Avatrombopag hydrochloride (0.3-3 mg/kg; p.o.; daily for 14 days) increases the number of human platelets in NOD/SCID mice transplanted with human FL CD34+ cells[1]. Animal Model: NOD/SCID mice (transplanted with human FL CD34+cells)[1] Dosage: 0.3, 1, and 3 mg/kg Administration: P.o.; daily for 14 days Result: Dose-dependently increased the number of human platelets, resulting in approximately a 2.7‐fold increase at 1 mg/kg/d and a 3.0-fold increase at 3 mg/kg/d on day 14 after the start of administration. |
| In Vitro: | Avatrombopag (E5501; AKR-501) hydrochloride specifically targets the TPO receptor and stimulated megakaryocytopoiesis throughout the development and maturation of megakaryocytes just as recombinant human TPO (rhTPO) did. Avatrombopag hydrochloride is showed to have effect in humans and chimpanzees only[1]. Avatrombopag hydrochloride (0-100 nM) supports the proliferation of TPO receptor expressing Ba/F3 cell in a concentration-dependent fashion. Avatrombopag hydrochloride (0-3 μM) induces tyrosine phosphorylation of STAT3 and STAT5, and threonine phosphorylation of ERK in the cells, as did rhTPO[1]. Avatrombopag hydrochloride promotes megakaryocyte colony formation from human CB CD34+ cells in a concentration-dependent fashion. The EC50 is 25 nM for Avatrombopag hydrochloride and the maximum activity of Avatrombopag hydrochloride is similar to that of rhTPO[1]. |
| References: | [1]. Fukushima-Shintani M, et al. AKR-501 (YM477) a novel orally-active thrombopoietin receptor agonist. Eur J Haematol. 2009;82(4):247-254. [2]. Xu H, et al. Avatrombopag for the treatment of thrombocytopenia in patients with chronic liver disease. Expert Rev Clin Pharmacol. 2019 Sep;12(9):859-865. [3]. Nomoto M, et al. Pharmacokinetic/pharmacodynamic drug-drug interactions of avatrombopag when coadministered with dual or selective CYP2C9 and CYP3A interacting drugs. Br J Clin Pharmacol. 2018;84(5):952-960. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.