| DC82301 |
IC-8
|
IC8 is an ionizable cationic lipid. It has been used in combination with other lipids for the formation of lipid nanoparticles (LNPs). Immunization with severe acute respiratory coronavirus 2 (SARS-CoV-2) spike glycoprotein mRNA in IC8- and manganese-containing LNPs induces IgG responses to SARS-CoV-2 Delta and Omicron variants in mice.1 Administration of mRNA encoding B7-H3 X CD3 bispecific T cell engaging (BiTE) antibodies in IC8-containing LNPs reduces tumor growth in MV4-11 and A375 mouse xenograft models. |
| DC46471 |
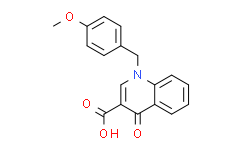
RP101988
|
RP101988, the major active metabolite of Ozanimod, is a selective, potent S1PR1 (sphingosine-1-phosphate receptor 1) agonist, with EC50s of 0.19 nM and 32.8 nM for S1PR1 and S1PR5, respectivlely. |
| DC37901 |
PD-173212
|
PD-173212 is a small molecule N-type calcium channel blocker. |
| DC37333 |
N,N-Diethyl-p-toluamide
|
N,N-Diethyl-p-toluamide is a mosquito repellent. |
| DC37321 |
AI3-15902
|
AI3-15902 is a biochemical. |
| DC37283 |
Methyl phenylcarbamate
|
Methyl phenylcarbamate is a biochemical. |
| DC37252 |
Ampyrone
|
Ampyrone is a metabolite of AMINOPYRINE with analgesic and anti-inflammatory properties. It is used as a reagent for biochemical reactions producing peroxides or phenols. Ampyrone stimulates LIVER MICROSOMES and is also used to measure extracellular water. |
| DC37245 |
Piperonyl butoxide
|
Piperonyl butoxide (PBO) is an organic compound used as a component of pesticide formulations. It is a waxy white solid. It is a synergist. That is, despite having no pesticidal activity of its own, it enhances the potency of certain pesticides such as carbamates, pyrethrins, pyrethroids, and rotenone.[1] It is a semisynthetic derivative of safrole. |
| DC36443 |
DC-Chol
|
DC-Chol is a cationic cholesterol derivative. DC-Chol, as a component of lipoplexes with DOPE, has been used for transfection of mRNA into A549 cells without affecting cell viability. Incubation of DC-chol/DOPE liposomes or lipoplexes with human whole blood has no effect on neutrophil elastase or β-thromboglobulin levels or the number of platelets and red and white blood cells, indicating hemocompatibility. DC-Chol has also been widely used in the synthesis of liposomes for the delivery of siRNA, DNA, and chemotherapeutic agents into cells and mice. |
| DC34632 |
MDL800
|
MDL-800 is a first-in-class cellularly active SIRT6 allosteric activator. |