| Cas No.: | 155206-00-1 |
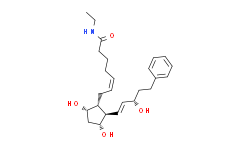
| Chemical Name: | (Z)-7-[(1S,2S,3R,5R)-3,5-Dihydroxy-2-[(E)-3-hydroxy-5-phenylpent-1-enyl]cyclopentyl]-N-ethylhept-5-enamide |
| Synonyms: | 17-PHENYL TRINOR PROSTAGLANDIN F2ALPHA ETHYL AMIDE;BIMATOPROST;BIMATOPROST(TM);LUMIGAN;N-ETHYL-9ALPHA,11ALPHA,15S-TRIHYDROXY-17-PHENYL-18,19,20-TRINOR-PROSTA-5Z, 13E-DIEN-1-AMIDE;BIMATOPROST(LUMIGAN);17-phenyl-tri-norprostaglandin f2α-ethyl amide;(Z)-7-[(1R,2R,3R,5S)-3,5-Dihydroxy-2-[(E,3S)-3-hydroxy-5-phenylpent-1-enyl]cyclopentyl]-N-ethylhept-5-enamide;(5Z)-7-[(1R,2R,3R,5S)-3,5-Dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenyl-1-penten-1-yl]cyclopentyl]-N-ethyl-5-heptenamide;Prostamide;C25H37NO4;17-phenyl trinor Prostaglandin F2α ethyl amide;AGN 192024;Bimatoprost (17-Phenyl-tri-norprostaglandin F2α-ethyl amide);BiMapnost;BiMatoprost HOUSE STANDARD;Bimatoprost API;cGMP Bimatoprost;Bimatoprost, >=99.5%;(15R)-BiMatoprost;(Z)-7-[(1S,2S,3R,5R)-3,5-Dihydroxy-2-[(E)-3-hydroxy-5-phenylpent-1-enyl]cyclopentyl]-N-ethylhept-5-e;(Z)-7-[(1S,2S,3R,5R)-3,5-Dihydroxy-2-[(E)-3-hydroxy-5-phenylpent-1-enyl]cyclopentyl]-N-ethylhept-5-enamide |
| SMILES: | O([H])[C@]1([H])C([H])([H])[C@]([H])([C@@]([H])(/C(/[H])=C(\[H])/C([H])(C([H])([H])C([H])([H])C2C([H])=C([H])C([H])=C([H])C=2[H])O[H])[C@]1([H])C([H])([H])/C(/[H])=C(/[H])\C([H])([H])C([H])([H])C([H])([H])C(N([H])C([H])([H])C([H])([H])[H])=O)O[H] |
| Formula: | C25H37NO4 |
| M.Wt: | 415.5656 |
| Purity: | >99% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Bimatoprost is a prostaglandin analog used topically (as eye drops) to control the progression of glaucoma and in the management of ocular hypertension. |
| References: | [1]. Park J, et al. Changes to upper eyelid orbital fat from use of topical bimatoprost, travoprost, and latanoprost. Jpn J Ophthalmol. 2011 Jan;55(1):22-7. [2]. Filippopoulos T, et al. Periorbital changes associated with topical bimatoprost. Ophthal Plast Reconstr Surg. 2008 Jul-Aug;24(4):302-7. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.