| Cas No.: | 4449-51-8 |
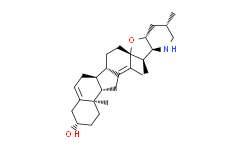
| Chemical Name: | Cyclopamine |
| Synonyms: | Cyclopamine;11-Deoxojervine;(3b,23b)-17,23-Epoxy-11-deoxoveratraman-3-ol;Cyclopamine (1-Deoxojervine);(2′R,3S,3′R,3′aS,6′S,6aS,6bS,7′aR,11aS,11bR)-1,2,3,3′a,4,4′,5′,6,6′,6a,6b,7,7′,7'a,8,11,11a,11b-octadecahydro-3′,6′,10,11b-tetramethyl-spiro[9H-benzo[a]fluorene-9,2′(3′H)-furo[3,2-b]pyridin]-3-ol;(3β,?23R)-?17,?23-?epoxyveratraman-?3-?ol;(3β,23R)-17,23-Epoxyveratraman-3-ol;11-deoxo-jervin;11-DEOXYJERVINE;11-Desoxo-jervin;alkaloidv;deoxojervine;ZH658AJ192;Jervine, 11-deoxo-;CY8 |
| SMILES: | O1[C@]2([H])C([H])([H])[C@]([H])(C([H])([H])[H])C([H])([H])N([H])[C@@]2([H])[C@@]([H])(C([H])([H])[H])[C@@]21C(C([H])([H])[H])=C1C([H])([H])[C@]3([H])[C@@]4(C([H])([H])[H])C([H])([H])C([H])([H])[C@@]([H])(C([H])([H])C4=C([H])C([H])([H])[C@@]3([H])[C@]1([H |
| Formula: | C27H41NO2 |
| M.Wt: | 411.6199 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Cyclopamine is a Hedgehog (Hh) pathway antagonist with an IC50 of 46 nM in the Hh cell assay. |
| In Vivo: | Cyclopamine causes durable regression of xenograft tumors. Tumors in Cyclopamine-treated animals, regress completely by 12 days[2]. Cyclopamine (1.2 mg) treatment blocks tumour formation of human pancreatic adenocarcinoma cells after transplantation into nude mice[3]. |
| In Vitro: | Treatment with small molecule Hh inhibitors such as HhAntag and the natural product Cyclopamine, both binding to Smo, induces tumor remission in a genetic mouse model of medulloblastoma[1]. Cyclopamine is a Hedgehog (Hh) pathway antagonist. Cyclopamine suppresses cell growth. Cyclopamine (3 μM) suppression of Hh pathway activity and growth in digestive tract tumour cell lines correlates with expression of PTCHmRNA[2]. Cyclopamine is a steroidal alkaloid that inhibits Hh signalling through direct interaction with Smo[3]. |